Anti-Saccharomyces cerevisiae antibodies, anti-mannan Candida antibodies and fungal colonization of the gastrointestinal tract in patients with ulcerative colitis, Crohn’s disease and irritabl
Dorota Ksiądzyna, Jarosława Semianów-Wejchert, Urszula Nawrot, Katarzyna Włodarczyk
+ Pracoviště
Souhrn
Protilátky proti Saccharomyces cerevisiae, protilátky proti mannanové složce Candidy a kolonizace gastrointestinálního traktu plísněmi u nemocných s ulcerózní kolitidou, Crohnovou chorobou a dráždivým tračníkem
Cíl studie: Role plísní v etiopatogenezi zánětlivých střevních chorob (IBD) nebyla dosud extenzivně studována. Účelem studie bylo stanovení protilátek proti Saccharomyces cerevisiae (ASCA), protilátek proti mannanové složce Candidy (AMCA) a stanovení kolonizace gastrointestinálního traktu plísněmi u těchto chorob ve srovnání s dráždivým tračníkem.
Materiál a metodika: Sérové hladiny ASCA a AMCA byly stanoveny ELISA technikou u 42 nemocných s ulcerózní kolitidou (UC), 26 nemocných s Crohnovopu chorobou (CD) a 19 nemocných s dráždivým tračníkem (IBS). Kvalitativní a kvantitativní kultivace stolice na plísně byly provedeny u všech nemocných.
Výsledky: ASCA byly nalezeny u 57,69 % nemocných s CD, 2,38 % u nemocných s UC a u 36,84 % nemocných s dráždivým tračníkem (IBS). Kultivace stolice byla pozitivní u 24 CD, 36 UC a 14 IBS nemocných s převahou Candida albicans (83,3 %, 91,66 %, 71,42 % resp.) u všech skupin. Signifikantní kolonizace plísněmi (SFC) byla prokázána u 3 UC a 4 IBS pacientů.
Závěry: 1. ASCA má větší prevalenci u CD než u CD a IBS. 2. AMCA byly přítomny u menšiny IBD pacientů. 3. SCF byla nalezena pouze u UC a IBS. 4. AMCA a SCF byly častější u IBS než u IBD, což naznačuje, že plísně by mohly hrát úlohu v etiopatogenezi IBS.
Klíčová slova: protilátky pro mannanovcé složce Candidy, protilátky proti Saccharopmyces cerevisiae, plísně, zánětlivá onemocnění střevní, dráždivý tračník.
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are two clinically different types of inflammatory bowel disease (IBD) characterized by chronic, recurrent and tissue damaging inflammation of the gastrointestinal (GI) tract. Although the aetiology and pathogenesis of IBD are not fully understood, there is an evidence for immunomodulation disorders in the response to intestinal flora(1–3). Disturbed balance between proinflammatory and anti-inflammatory cytokines promotes long-standing active inflammation and subsequent tissue injury.
The idea that microorganisms play a role in the aetiology of IBD has gained ground considerably in recent years. The large number of infectious agents proposed to play a role in the development of CD and UC may partly be explained by alterations caused by these microorganisms to the normal intestinal flora. Thus extensive experimental and clinical data suggest that luminal bacteria or their products play a significant role in the initiation and perpetuation of chronic intestinal inflammation(4,5). Until recently microbiological studies have been focused on bacterial aspects in the pathogenesis of IBD, however in the last years an interest in the presence of fungi in the GI tract has increased. Nevertheless, number of published data dealing with yeasts is relatively scanty in comparison with research on bacterial pathogens in IBD.
Yeasts, mostly Candida spp. phylogenetically related to Saccharomyces spp., are important component of the human GI tract microflora. Although considered as a part of residual microbial system, Candida spp. are also a risk factor of oportunistic infections in immunocompromised patients. The antibacterial and immunosuppressive therapy used in the treatment of IBD may favour yeasts’ multiplication and development of mycoses, especially in the presence of mucosal lesions of the intestine. Moreover, Candida spp. are supposed to facilitate permeation of food antigens through the mucosal barrier of the GI tract with the involvement of mast cells via mediators like protease II or TNFα(6,7). There is also evidence for the deteriorating influence of Candida spp. on the healing process of inflammed colon in the animal model of UC(8) and positive effects of concomitant therapy with a probiotic (Lactobacillus acidophilus) and antifungal treatment with improved healing of colonic lesions, decreased colonic inflammation, attenuated metaloproteinase activity and plasma proinflammatory cytokines (IL-1β and TNF-α) levels as well as shortened duration of fungal colonization of the mucosa in humans(9).
Mechanisms of bacterial and fungal balance, still explored to a small extend, may play an important role in the pathogenesis of IBD. In patients with IBD antibodies against microorganisms of the intestinal flora are found more frequently than in healthy individuals, probably reflecting the increased immunoreactivity of the compromised gut. Antibodies against mannans of Saccharomyces cerevisiae (ASCA), antigens widely distributed in many food products, have been considered as an immunological marker characteristic for CD since 1988(10). The anti-mannan Candida antibodies (AMCA) against the mannan component of Candida albicans (C. albicans) cell wall are present in 18% of healthy children aged 3–10 years, 48% individuals aged 11–19 years and 76% of adults(11). Although the association between an elevated level of AMCA and development of candidiasis has been observed in some groups of patients, IBD and IBS (irritable bowel syndrome) patients have not been examined extensively so far(12). It seems that AMCA and the assessment of fungal colonization of the GI tract might be useful not only in diagnostics of often latent fungal infections but also in better understanding of the aetiopathogenesis of IBD.
Considering all these above-mentioned facts, the aim of this study was the assessment of the serum level of ASCA and AMCA and the fungal colonization of the lower part of the GI tract in patients with IBD and IBS.
MATERIAL AND METHODS
Patients and study design
Eighty seven consecutive patients with already established diagnosis of IBD or IBS hospitalized in the Department of Gastroenterology and Hepatology, Wrocław Medical University, Poland were subjected to the study. The studied group consisted of 42 patients with UC (32 female, 10 male; mean aged 43.05 ± 10.22 years), 26 patients with CD (15 female, 11 male; mean aged 34.64 ± 11.27 years) and 19 patients with IBS (10 female, 9 male; mean aged 45.0 ± 10.85 years). BMI in UC, CD nad IBS patients equalled 24.35 ± 3.96, 20.82 ± 3.31 and 25.29 ± 4.93 kg/m², respectively. Clinical data were gathered at the time of blood and stool sampling in all subjects. Clinical and endoscopic activity of UC was estimated according to Rachmilewitz’s index. Mean clinical activity equalled 6.51 ± 4.13 points, whereas endoscopic activity 5.22 ± 2.96 points. Thirty one patients suffered from active disease and 11 were in remission at the time of hospitalization. Erytrocyte sedimentation rate ranged from 4 to 120 (mean 22.4/l h). CRP was elevated (> 6.3 mg/L) in 8 subjects (7.0–84.4, mean 29.1) mg/L. In terms of disease duration, it ranged from 6 months to 20 years (mean 6.3 ± 5.67 years). At the time of the study the patients were receiving the following specific treatment: oral 5-aminosalicylate only: 29 subjects, 5-aminosalicylate together with oral prednisone: 10 subjects, oral 5-aminosalicylate and hydrocortisone hemisuccinate intravenously: 3 subjects.
As concerns the localization of the gut involvement, Crohn’s disease was limited to the terminal ileum in 5 patients whereas 12 subjects suffered from Crohn’s colitis and 9 from ileocolitis. All patients with previous history of abdominal surgery related to the IBD course or a stenosis or a fistula revealed on recent endoscopic or radiologic examination were not included in the study. Activity of CD was estimated according to Crohn’s Disease Activity Index (CDAI) and ranged between 89.2 and 297.5 points (mean: 264.0 ± 89.84) points. In 8 patients CDAI was below 150 points. Erytrocyte sedimentation rate ranged from 4 to 98 (mean: 27.0/l h) and CRP was elevated (> 6.3 mg/L) in 8 subjects (8.0 – 90.4, mean: 38.8) mg/L. Disease duration ranged from 1 year to 11 years (mean 4.43 ± 2.95 years). At the time of the study the patients were receiving the following specific treatment: oral 5-aminosalicylate only: 7 patients, oral 5-aminosalicylate together with oral prednisone: 12 patients, oral 5-aminosalicylate and hydrocortisone hemisuccinate intravenously: 2 patients, oral prednisone together with azathioprine: 3 patients, oral azathioprine only: 2 patients.
IBS patients, all with alternating bowel habit (episodes of diarrhoea and constipation in every patient), constitued a third group. All IBS subjects fulfilled the Rome II criteria which were in use at the time of the inclusion to the study protocol. Apart from medical history and physical examination, routine blood and stool tests, abdominal ultrasonography and a complete colonoscopy were performed together with investigations to exclude infectious and microscopic colitis, lactose intolerance, hyperthyroidism and coeliac disease (serum anti-tissue transglutaminase IgA, anti-endomysial IgA/IgG antibodies, total IgA and a microscopic examination of the small bowell biopsy specimens obtained on the endoscopy of the upper GI tract). In patients who failed to respond to reassurance, treatment tailored to the predominant symptoms was given on demand, but none of them has been taking drugs that could interfere with the results of the study performed. Exclusion criteria in both IBD and IBS patients comprised a history of an abdominal surgery affecting the anatomical integrity of the GI tract, diabetes mellitus and treatment with antibiotics or antifungal drugs during a month prior to the hospitalization.
Informed consent was given by every participant and the study protocol conformed to the Declaration of Helsinki was approved by the local Medical Ethics Committee at Silesian Piasts Medical University of Wrocław (agreement no. 412/2003).
ASCA detection
The level of ASCA was measured with the use of commercial enzyme-linked immunosorbent assay (ELISA) kit: Euroimmun (Medizinische Labordiagnostika AG, Lübeck, Germany) according to the manufacturer’s instructions. Briefly, patients sera were diluted 1 : 100 and added together with appropriate controls and calibrators on microplates, coated with purified mannan from the cell wall of S. cereviasiae (oligomannose sequences alpha-1,3 Man are antigens to ASCA). After incubation for 30 minutes at room temperature (18 to 25 °C) and washing 3 times, the peroxidase-labelled anti-human IgG were added into each of the microplate wells. Subsequently, after incubation for 30 minutes at room temperature, unbound antibodies were removed and chromogen solution was introduced. Photometric measurement of the colour intensity was performed at a wavelength of 450 nm and a reference wavelength of between 620 nm and 650 nm within 30 minutes of adding the stop solution. The results were expressed in relative units (U) with the value ≥ 20 U/mL considered as positive.
AMCA detection
The serum level of AMCA from fasting blood samples was measured with commercial ELISA kit Platelia Candida Ab (BioRad, France) according to the manufacturer’s instructions, which has already been described(13). To be brief, microplates coated with cell wall mannan from C. albicans VW32 were used in the test. Serum (100 μl) diluted 1/6500 was added to each well and the plates were incubated. After washing, peroxidase-conjugated anti-human immunoglobulins were added, which bound to the anti-mannan antibodies present in the serum. The immunological reaction was revealed by the development of a coloured enzymatic reaction with intensity proportional to the antibody concentration. A standard dilution curve allowed the determination of anti-mannan antibody concentration. The test detects total AMCA (IgG, IgM, IgA). The minimal detectable antibodies level was 2.5 U/mL.
Mycological stool examination
Mycological investigation of the stool was performed in all subjects, including the quantitative and qualitative fungal cultures. The stool samples (1–2 g) were treated with 0.03% tripsine solution and cultured quantitatively on Sabouraud agar plates supplemented with chloramphenicol. After 48–72 h of incubation at 37 and 28 °C the colonies were counted and subcultured on Chromagar Candida (Becton Dickinson, USA) and Rice media. Quantitative mycological investigation was performed according to Müller’s method(14). Isolates were identified basing on morphological features and biochemical tests (ID-32C, BioMerieux, France). Moulds were identified on the basis of their morphology. The results of at least 105 CFU/g (colony forming units per one gram of stool) were considered as significant fungal colonization (SFC).
Statistics
Obtained results were analysed statistically on the basis of Statistica Software 6.0 (Statsoft, Cracow, Poland). Data were analysed using a set of statistical tests (the Student’s t-test, the Mann-Whitney U test and the Spearman rank correlation coefficient, χ2 test and Fisher’s test) for parametric and nonparametric variables. P-values less than 0.05 were considered significant.
RESULTS
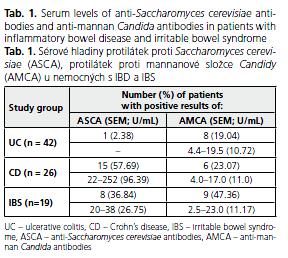
ASCA were found in 15 (57.69%) patients with CD and 1 (2.38%) patient with UC (Table 1). The levels of ASCA were significantly higher in CD than in UC and IBS patients (p = 0.002 and 0.003, respectively). There was no correlation between the serum level of ASCA and IBD activity. ASCA were also positive in 7 (36.84%) IBS patients ranging from 20.0 to 38.0 (mean 26.75) U/mL.
AMCA were observed in 8 (19.04%) subjects with UC, all in an active phase of the disease, and their levels ranged from 4.4 to 19.5 (mean 10.72) U/mL. No statistical differences of AMCA levels in UC patients with diverse disease activity were found (p > 0.05). AMCA were positive in 6 (23.07%) CD patients and their level ranged from 4.0 to 17.0 (mean: 11.0) U/mL. AMCA were positive in 9 (47.36%) IBS patients with the range from 2.5 to 23.0 (mean: 11.17) U/mL.
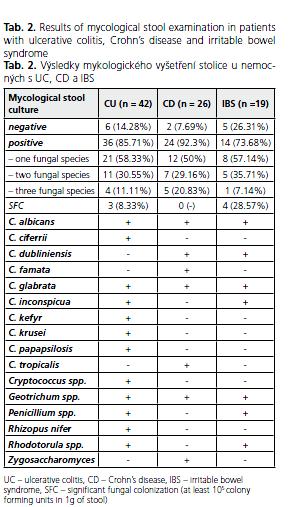
In the majority of patients with UC (85.71%) mycological examination showed the presence of fungi with the predominance of C. albicans, which was found in 33 (91.66%) subjects (Table 2). Other fungi were represented by C. glabrata, C. crusei, C. inconspicua, C. kefyr, C. parapsilosis, C. ciferrii, rarely species other than Candida like Geotrichum spp. (6 patients), Rhodotorula spp. (1 patient), Rhizopus nifer (1 patient), Cryptococcus spp. (1 patient) and Penicillium spp. (1 patient). However, SFC was revealed in only 3 (8.33%) UC patients. All subjects with SFC suffered from active UC with clinical activity index ranging from 9 to 12 (mean 10.33) points. In 2 patients with SFC C. albicans was cultured, whereas in the third one 2 fungal species were found in the stool sample ( C. albicans and Geotrichum spp.). Similarily, C. albicans disclosed in 20 (83.33%) subjects and Candida spp. ( C. dubliniensis, C. glabrata, C. tropicalis, C. famata) were the most frequently cultured fungi in 24 CD patients with positive fungal culture of the stool. Geotrichum spp. were found in 2 subjects and Zygosaccharomyces spp. in one of them. However, SFC was revealed in none of CD patients. Positive fungal culture was found in 14 (73.68%) IBS patients with SFC observed in 4 of them (28.57%). One fungal species was obtained in 8, two in 5 and three in one of them. Likewise in IBD, C. albicans was the dominant genius (in 10 subjects), C. glabrata was found in two, C. inconspicua in one and C. dubliniensis in one patient. There there also Geotrichum spp. (4), Pencillium spp.(1) and Rhodotorula spp.(1).
There were no significant differences between IBD patients during steroid therapy or without steroid therapy as far as AMCA level and SFC are concerned (p ≤ 0.05). There was also no correlation between AMCA levels, SFC and disease duration in this group of subjects.
DISCUSSION
In our study we focused on the levels of circulating ASCA and AMCA as well as fungal colonization of the the GI tract, which have risen large interest in IBD and IBS.
Alterations of the bacterial microflora are regarded as an important factor triggering the disease process in IBD and may explain the efficacy of antibiotic treatment in active phase of the disease, especially UC. Fungal overgrowth may be the complication of any bacterial imbalance, e.g. due to antibiotic therapy. Disturbed relationship of bacterial and fungal species in the mucosal microflora reflects a metabolic imbalance of the complex microbial ecosystem with pathophysiological consequences for mucosal barrier.
The presence of yeasts and their mannans, which are potent IFN-α inductors may predispose to disease exacerbations and superinfections of mucosal lesions. Moreover, the gut may be the source of dissemination. Factors predisposing to candidiasis, often found in IBD patients include broad-spectrum antibiotics or steroid treatment, intravascular catheters and intravenous alimentation. The diagnosis is difficult due to nonspecific clinical signs and the natural commensal status of these oportunistic pathogens.
Previous reports have suggested that the combined serological detection of mannanemia and AMCA may be useful for the diagnosis of systemic candidiasis caused by C. albicans and serological surveillance of at-risk patients could result in earlier initiation of antifungal therapy(15,16). However, anti-mannan antibodies have been shown to be ubiquitous in human sera, presumably because the immune system can be stimulated as a result of colonization by C. albicans in the absence of the disease(17,18). Patients with Candida spp. mucosal infections or with asymptomatic fungal colonization may also have positive results of the test. According to data provided by the manufacturer of the test titres ranging from 2.5 to 5.0 are detected in hospitalized patients both colonized and uncolonized by Candida spp. (11.8% and 9.1%, respectively; 11.8% with invasive candidiasis), titres between 5.0–10.0 U/mL in 3% of uncolonized and 31.4% of colonized (28.8% with invasive candidiasis) and titres higher than 20.0 U/mL in 3% of uncolonized and 13.7% of colonized patients (9.6% with invasive candidiasis). Given that AMCA are ubiquitous in human sera, serological tests often fail in discrimination between colonization and invasive candidiasis, leading therefore to poor specificity values (false positives). Furthermore, their low sensitivity (false negatives) registered in severily immunosuppressed patients could also be due to a delayed, reduced or an absent immunological response. Platelia Candida Ab test is said to have specificity and sensitivity of 94% and 53%, respectively(13).
The presence of C. albicans-specific IgG antibodies in serum samples and the presence of C. albicans in stool samples did not exhibit any significant difference between 21 patients in active stage and 15 patients in remission of UC(19), which is in agreement with the results of our study. In another study AMCA were determined and followed in 57 UC patients(20). The appearance of higher titers entailed an intermittent (benign) clinical course of the disease, whereas lower titer values at repeated checks were associated with a continuous (severe) clinical course. The authors suggest that AMCA titer values may be used as an indicator of the clinical outcome. On the basis of our research, such conclusion cannot be neither proved nor denied, as we did not perform a serial measurements of AMCA.
Prevelance of Candida spp. and C. albicans carriage equalls 53.4% and 46.5%, respectively, indicating frequent commensal carriage(21). Multilocus sequence typing of C. albicans isolates revealed frequent colonization of a subject or several members of the same family by genetically indistinguishable or genetically close isolates.
Zwolińska-Wcisło M. at al. found that SFC was more frequent in patients with UC history over 5 years in comparison with shorter disease duration and IBS (33.3%, 13.8% and 1.3%, respectively)(22). Likewise our research, mycological examinations revealed that C. albicans was the most frequent fungus isolated from the colon (91.7%) with other yeasts (C. glabrata - 6.7%, C. incospicua – 1.6%) being less predominant. Like in our study, initial analysis of total mean activity indices of inflammation in UC patients with significant and insignificant fungal colonization of the colon did not demonstrate sigificant differences between these groups. However, after 4 weeks stronger decrease of the UC activity index was observed in patients with SFC treated with antifungal drugs in comparison with patients not given antifungal therapy. The authors claim that SFC may exacerbate UC, longer history of the disease is a risk factor of SFC and antimycotic treatment could ameliorate clinical picture of UC. In contrast to the study we did not show that SFC was more prevalent in UC patients with a disease history longer than 5 years, although one of our subjects has been suffering from UC for 16 years (the remaining two for 1 and 4 years). However, number of subjects with SFC was low in our investigation, so it is difficult to compare the results.
The presence of IgG antibody to S. cerevisiae is characteristic but not specific to CD. In the majority of studies ASCA are detected in 50–80% of CD patients and less than 10% of UC, which is a result similar to our study (57.69% and 2.38%, respectively)(23–26). However, measurements of ASCA and AMCA serum levels differs according to the method used thus the results published by different authors are not always comparable. ASCA tests are not standardised and different methods for ASCA detection exist(27,28). It is estimated that sensitivity of ASCA detection in CD ranges from 39% to 71% and specificity is about 90%(29,30). Like in the majority of studies no correlation between sex, age, localization of inflammatory leasions in the GI tract and presence of ASCA was found(31–34).
Interpretation of the results becomes more difficult when we realise that a mannan component of cellular wall is present both in Candida spp. and Sacccharomyces spp. Some authors claim that there is at least a partial cross-reactivity of these antigens(35,36). IgG serum antibody was measured by ELISA in CD, UC and in normal controls (15 subjects in each group) to 12 strains of S. cerevisiae and to the two major serotypes of the commensal C. albicans(37). Antibody to 11 of the 12 strains of S. cerevisiae was raised in patients with CD but not in UC. The pattern of antibody response to these 11 strains was variable, however, suggesting the likelihood of antigenic heterogeneity within the species. AMCA was not significantly different in patient and control groups. Structural and genetic studies have shown that C. albicans produces mannosyltransferase enzymes that can synthesize S. cerevisiae oligomannose sequences depending on growth conditions. Standaert-Vitse A. et al. revealed that in humans and rabbits, generation of ASCA was shown to be associated with the generation of AMCA resulting specifically from infection(36). By using affinity-purified antibodies, C. albicans was shown to express ASCA epitopes on mannoproteins similar to those of S. cerevisiae. By changing the growth conditions, C. albicans mannan was also able to mimic S. cerevisiae mannan in its ability to detect ASCA associated with CD. This overexpression of ASCA epitopes was achieved when C. albicans grew in human tissues. During C. albicans infection ASCA are generated, which dissapear in the majority of individuals except for CD patients. C. albicans is one of several immunogens for ASCA and may be at the origin of an aberrant immune response not only in CD but also in IBS. ASCA and AMCA were revealed in as many as 36.84% and 47.36% of IBS patients, respectively, in our study. Higher frequency of ASCA detection in comparison with other studies(5,38) is an observation difficult to explain. It is wildly known that IBS encompasses a wide range of symptoms with abdominal pain and an altered bowel habit as a typical clinical presentation. However, these are joint clinical features for IBS and most cases of IBD, especially CD with mild clinical activity. There is also evidence that high levels of some "new" serological IBD biomarkers like anti-laminaribioside IgG (ALCA) and AMCA are significantly associated with small intestinal disease(39). Our IBS group consisted of patients with the final diagnosis established by the treating gastroenterologist based on signs, symptoms and a standard set of examinations (also tests to exclude coeliac disease) undertaken in such cases in our department. However, two facts may impede the clear interpretation of these results. Firstly, it has been shown that sensitivity and specificity of positive Rome II criteria fulfilled by all our patients were not perfect (85% and 81%, respectively) in the differential diagnosis of organic vs. functional intestinal disease(40). Secondly, the decision to perform any type of visual examination of the jejunum and the proximal ileum was the independent decision of the treating gastroenterologist and one of the inclusion criteria in our study was full colonoscopy, but not ileo-colonoscopy. Therefore, despite the fact that some IBS patients underwent terminal ileum intubation during colonoscopy, in some other patients CD of mild clinical activity limited to terminal ileum might be missed and patient’s complaints were referred to IBS. It has been reported that the IBS-like symptoms may proceed the final diagnosis of IBD(41). Possibly, these unintentionally misdiagnosed cases of Crohn’s ileitis could be responsible for the higher frequency of ASCA and relatively high levels of AMCA in our study.
Some patients with IBS develop symptoms following an episode of gastroenterititis(42). It has been well documented that antibiotics administered in case of severe gastrointesitnal infections cause a disbalance of gut microflora, favour fungal overgrowth and may be a risk factor of post-infectious IBS(43). Hypothetically, increased yeasts’ multiplication with abberant immune response might be responsible for both higher frequency of AMCA and the failure of clinical trials with high-dose steroid therapy in post-infectious IBS with minimal inflammation confirmed on microscopic examination of colonic mucosa, however there is a need for in-depth studies to explain this observation.
CONCLUSION
Results of our study show that:
1. ASCA are more prevalent in CD than in UC and IBS.
2. Mean serum level of ASCA is significantly higher in CD than in UC and IBS.
3. SFC is found in UC and IBS, but not CD.
4. C. albicans and other yeasts of the species Candida are the most predomiant component of stool cultures in both IBD and IBS patients.
5. AMCA and SFC are present in the minority of patients with CD and UC.
6. AMCA are detected more often in IBS than IBD patients.
7. Relatively high prevalence of AMCA and SFC of the lower part of GI tract in patients with IBS suggest that yeasts might play a role in the pathogenesis of this functional disorder. However, this observation requires further studies on a bigger number of patients.
Acknowledgements
Declaration of personal interests: None. Declaration of funding interests: This study was supported by a grant from the Silesian Piasts Medical University of Wrocław, Wrocław, Poland (protocol number: 905).
References
- 1. Anand A, Adya C. Cytokines and inflammatory bowel disease. Trop Gastroenterol 1999; 20: 97–106.
- 2. Papadakis K, Targan S. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med 2000; 51: 289–298.
- 3. Schreiber S. Inflammatory bowel disease: immunologic concepts. Hepatogastroenterology 2000; 47: 15–28.
- 4. Duchmann R, Kaiser I, Hermann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease IBD; Clin Exp Immunol 1995; 102: 448–455.
- 5. Schoepfer A, Schaffer T, Seibold-Schmid B, et al. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol Motil 2008; 30: 1110–1118.
- 6. Yamaguchi N, Sugita R, Miki A, et al. Gastrointestinal Candida colonization promotes sensitisation against food antigens by affecting the mucosal barrier in mice. Gut 2006; 55: 954–960.
- 7. Benjamin J, Makharia G, Joshi Y. Association between intestinal permeability and anti-Saccharomyces cerevisiae antibodies in patients with Crohn’s disease. Inflamm Bowel Dis 2008; 14: 1610–1611.
- 8. Zwolińska-Wcisło M, Brzozowski T, Budak A, et al. Studies on the influence of Candida fungal colonization on the healing process of inflammatory lesions in the colon in rat animal model. Przegl Lek 2007; 64: 124–129.
- 9. Zwolińska-Wcisło M, Brzozowski T, Mach T, et al. Are probiotics effective in the treatment of fungal colonization of the gastrointestinal tract? Experimental and clinical studies. J Physiol Pharmacol 2006; 57(Suppl 9): 35–49.
- 10. Main J, McKenzie H, Yeaman GR, et al. Antibody to Saccharomyces cerevisiae baker’s yeast; in Crohn’s disease. BMJ 1988; 297: 1105–1106.
- 11. Faux JA, Agbarakwe AE, Misbah SA, et al. A comparison of specific IgG antibody levels to the cell wall mannan of Candida albicans in normal individuals and in patients with primary antibody deficiency. J Immunol Methods 1992; 153: 167–172.
- 12. van Deventer AJ, Goessens WH, van Zeijl JH, et al. Kinetics of anti-mannan antibodies useful in confirming invasive candidiasis in immunocompromised patients. Microbiol Immunol 1996; 40: 125–131.
- 13. Sendid B, Tabouret M, Poirot JL, et al. New enzyme immunoassays for sensitive detection of circulating Candida albicans mannan and antimannan antibodies: useful combined test for diagnosis of systemic candidiasis. J Clin Microbiol 1999; 37: 1510–1517.
- 14. Müller J. Fungi in the gastrointestinal trakt. Fortschr Med 1982; 100: 936–941.
- 15. Yera H, Sendid B, Francois N, et al. Contribution of serological tests and blood culture to the early diagnosis of systemic candidiasis. Eur J Clin Microbiol Infect Dis 2001; 20: 864–870.
- 16. Sendid B, Poirot JL, Tabouret M, et al. Combined detection of mannanaemia and antimannan antibodies as a strategy for the diagnosis of systemic infection caused by pathogenic Candida species. J Med Microbiol 2002; 51: 433–442.
- 17. López-Ribot JL, Casanova M, Murgui A, et al. Antibody response to Candida albicans cell wall antigens. FEMS Immunol Med Microbiol 2004; 41: 187– 196.
- 18. Martinez JP, Gil ML, López-Ribot JL, et al. Serologic response to cell wall mannoproteins and proteins of Candida albicans. Clin Microbiol Rev 1998; 11: 121–141.
- 19. Kalkanci A, Tuncer C, Degertekin B, et al. Detection of Candida albicans by culture, serology and PCR in clinical specimens from patients with ulcerative colitis: re-evaluation of an old hypothesis with a new perspective. Folia Microbiol 2005; 50: 263–267.
- 20. Nagy F, Szenasi Z, Szöllösy E, et al. Determination and kinetics of antibody response to Candida albicans in ulcerative colitis. Hepatogastroenterology 1989; 36: 182–184.
- 21. Bougnoux ME, Diogo D, Francois N, et al. Multilocus sequence typing reveals intrafamilial transmission and microevaluations of Candida albicans isolates from the human digestive tract. J Clin Microbiol 2006; 44: 1810–1820.
- 22. Zwolińska-Wcisło M, Budak A, Trojanowska D, et al. The influence of Candida albicans on the course of ulcerative colitis. Przegl Lek 2006; 63: 533–538.
- 23. Zawadzka P, Szczepański M, Łykowska-Szuber L, et al. The role of autoantibodies in diagnosing and identifying inflammator bowel diseases. Alergia Astma Immunol 2006; 11: 155–161.
- 24. Peeters M, Joossens S, Vermeire S, et al. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol 2001; 96: 730– 734.
- 25. Preda C, Diculescu M, Mirea V, et al. Significance of anti-Saccharomyces cerevisiae antibodies ASCA; in patients with inflammatory bowel diseases in Romania. Rom J Gastroenterol 2005; 14: 23–26.
- 26. Halme L, Turunen U, Helio T, et al. Familial and sporoadic inflammatory bowel disease: comparison of clinical features and serological markers in a geneticaly homogenous population. Scand J Gastroenterol 2002; 37: 692–698.
- 27. Klebl FH, Bataille F, Hofstadter F, et al. Optimising the diagnostic value of anti-Saccharomyces cerevisiae antibodies ASCA; in Crohn’s disease. Int J Colorectal Dis 2004; 19: 319–324.
- 28. Sandborn WJ, Loftus EV Jr, Colombel JF, et al. Evaluation of serologic disease markers in a population–based cohort of patients with ulcerative colitis and Crohn‘s disease. Inflamm Bowel Dis 2001; 7: 192–201.
- 29. Gupta S, Fitzgerald J, Croffie J, et al. Comparison of serological markers of inflammatory bowel disease with clinical diagnosis in children. Inflamm Bowel Dis 2004; 10: 240–244.
- 30. Kim J, Kim K, Seo J. Diagnostic role of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies in pediatric inflammatory bowel disease. Korean J Gastroenterol 2003; 42: 297–302.
- 31. Lerner A, Shoenfeld Y. Serological markers in inflammatory bowel disease: the pros and cons. Eur J Gastroenterol Hepatol 2002; 14: 103–105.
- 32. Vermeire S, Peeters M, Vlietinck R, et al. Anti-Saccharomyces cerevisiae antibodies ASCA; phenotypes of IBD, and intestinal permeability: a study in IBD families. Inflamm Bowel Dis 2001; 7: 8–15.
- 33. Vermeire S, Joosens S, Peeters M, et al. Comparative study of ASCA Anti–Saccharomyces cerevisiae antibody; assays in inflammatory bowel disease. Gastroenterology 2001; 120: 827–883.
- 34. Kountroubakis I, Petinaki E, Mouzas I, et al. Anti-Saccharomyces cerevisiae mannan antibodies and antineutrophil cytoplasmic autoantibodies in Greek patients with inflammatory bowel disease. Am J Gastroenterol 2001; 96: 449–454.
- 35. Nermes M, Savolainen J, Kortekangas-Savolainen O. Nitrocellulose-RAST analysis of allergenic crossreactivity of Candida albicans and Saccharomyces cerevisiae mannans. Int Arch Allergy Immunol 1995; 106: 118–123.
- 36. Standaert-Vitse A, Jouault T, Vandewalle P, et al. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn‘s disease. Gastroenterology 2006; 130: 1764–1775.
- 37. McKenzie H, Main J, Pennington CR, et al. Antibody to selected strains of Saccharomyces cerevisiae baker’s and brewer’s yeast; and Candida albicans in Crohn’s disease. Gut 1990; 31: 536–538.
- 38. Schoepfer A, Trummler M, Seeholzer P, et al. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis 2008; 14: 32–39.
- 39. Li X, Conlin L, Alex P. New serological biomarkers of inflammatory bowel disease. World J Gastroenterol 2008; 14: 5115–5124.
- 40. Tibble J, Sigthorsson G, Foster R, et al. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 2002; 123: 450–460.
- 41. Bercik P, Verdu E, Collins S, et al. Is irritable bowel syndrome a low grade inflammatory bowel disease? Gastroenterol Clin North Am 2005; 34: 235–245.
- 42. Barbara G, De Giorgio R, Stronghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol The r2004; Suppl 2: 1–9.
- 43. Barbara G, Stronghellini V, Cremon C, et al. Almost all irritable bowel syndromes are post–infectious and respond to probiotics: controversial issues. Dig Dis 2007; 25: 245–248.
Pro přístup k článku se, prosím, registrujte.
Výhody pro předplatitele
Výhody pro přihlášené