Hepatocellular carcinoma in central Slovakia – tertiary referral centre experience with 207 patients
Ľubomír Skladaný Orcid.org , Svetlana Adamcová-Selčanová1, Vladimír Malec2, Jana Badinková1, Eva Pritzová2, Július Janek3, Stanislav Okapec4
+ Affiliation
Summary
Introduction: The features of hepatocellular carcinoma (HCC) differ between geographical regions. Aim: To analyze a cohort from a tertiary referral centre. Methodology: The study employed a retrospective analysis of consecutive outpatients. The inclusion criterion was patients with HCC and the exclusion criterion was insufficient data. The study interval was 2007–2016. Results: Cohort 207 patients, 95% with cirrhosis, 76% men; age 62 years. Etiology of liver disease: Alcoholic 106 (48%), hepatitis C 17%, hepatitis B 13%, non-alcoholic steatohepatitis 10%, cryptogenic 7%, non-cirrhotic 4%, other 1%. Diagnosis by surveillance 24%, otherwise 67%, unknown 9%. Diameter if diagnosis by SUR-HCC 5 cm, otherwise 8.6 cm (p = 0,001). BCLC stages: A – 30 patients (15%), B – 29%, C – 36%, D – 20%; BCLC according to diagnosis (SUR-HCC vs. otherwise): A – 15/49 (31%) vs. 12/140 (9%), B – 43 vs. 27%, C – 16 vs. 39%, D – 10 vs. 25%. Treatment: Surgical resection in 17 patients (7%); liver transplantation 14 (6%); radiofrequency ablation 20 (8%); transarterial chemoembolization 60 (24%); sorafenib 88 (35%); best of supportive care 46 (19%), 3 patients were awaiting liver transplantation. Mean survival 17 (0.06–112) months; survival according to BCLC: A – 37 (2–112), B – 21 (0.6–86), C – 14 (0.06–92), D – 3 (0.06–24). Conclusion: The demographics in our cohort resembled those of Western Europe and North America. Alcoholic liver disease was the most common etiology for underlying liver disease; 95% of patients had cirrhosis. Only 24% of cases were detected by SUR-HCC; therefore, a marked shift to the right in the BCLC stage distribution was seen, what resulted in a suboptimal allocation of curative treatments and subsequently to worse survival. The treatment results and the management of HCC in our region are comparable with standard of care in West Europe and North America.
Keywords
diagnosis, hepatocellular carcinoma, cohort, treatment, survival, surveillanceIntroduction
Hepatocellular carcinoma (HCC) is the seventh most common cancer and the third leading cause of cancer-related death worldwide, with an estimated 748,000 new liver cancer cases and 696,000 liver cancer deaths caused in 2008 [1]. Its incidence ranks from the fifth most common cancer in men to the nineth most common cancer in women, and it is the fastest increasing cause of cancer-related death. The main causes of HCC are alcoholic liver disease (ALD), non-alcoholic steatohepatitis (NASH) and hepatitis C virus (HCV) [2–9].
Slovakia is one of the countries with low-to-medium incidence, with 3 to 30 new cases per 100,000 inhabitants; these countries are typical by the rise of incidence of HCC [1,7,10–25]. HCC is becoming the most frequent decompensating event in patients with previously compensated advanced chronic liver disease (ACLD), and in Slovakia it is one of the leading causes of liver transplantation (LTx; 10% of all LTx) [26,27]. Thanks to cross-sectional studies it is now possible to better detect demographic and clinical characteristics of HCC, which allows better planning of effective treatment strategies [19]. The expenses to society per patient with HCC are enormous – 32,907 USD; 89% of them are healthcare-related and 11% related to loss of productivity [14,28].
Risk factors of HCC
Most of the HCC cases (80–90%) arise in the context of liver cirrhosis, nowadays termed ACLD [10]. Other risk factors are usually intertwined so that their individual impacts over ACLD is quite difficult to quantify [29–31]. There are region-dependent risk factors, such as HBV, HCV, alcohol consumption and obesity; and region-independent (or at least less dependent) risk factors such as male gender, and being over 65 years of age. The structure of these factors should be known and considered. For example, there is recent evidence to suggest that there is increase in the incidence of HCC cases without underlying cirrhosis, especially in the association with non-alcoholic fatty liver disease (NAFLD), and the signal (small and questioned) of the increase in the incidence of HCC after the (successful) HCV infection therapy with interferon-free regimens [29,31–33].
Surveillance of HCC
When HCC manifests, the size of the tumour is usually > 5 cm, and the possi-bility of curative treatments and prognosis are poor. On the other hand, if at the time of diagnosis the tumour diameter is < 5 cm, curative modalities can be applied (radiofrequency catheter ablation – RFA, surgical resection – SR and LTx), and the probability of 5-year survival > 50% can be expected [34,35]. The most effective method of early diagnosis is SUR-HCC [36–39]. Guidelines-endorsed SUR-HCC modality is abdominal ultra-sonography (USG), performed by an ex-perienced physician every 6 months [5,30,31,40–42]. The effectiveness of SUR-HCC varies in dependence on region and etiology of ACLD – from 43 to 86% [32,43]. An average diameter of lesions detected by SUR-HCC as well as linkage to care can be consider-ed as the quality control measures which are recommended by guidelines [32,40,44–51].
Recall policy
The next step after detecting a new lesion by SUR-HCC is either computed tomography, or magnetic resonance imaging, or both [36–39,52]. The biopsy of tumour is usually performed in case of diagnostic uncertainty, or (in our region) for research; in this case, a biopsy of surrounding parenchyma could be considered in order to appreciate the so-called field effect [53]. After the publication of guidelines permitting non-invasive diagnosis of HCC, the use of liver biopsy had fallen to around 20% [54].
Prognostic classification
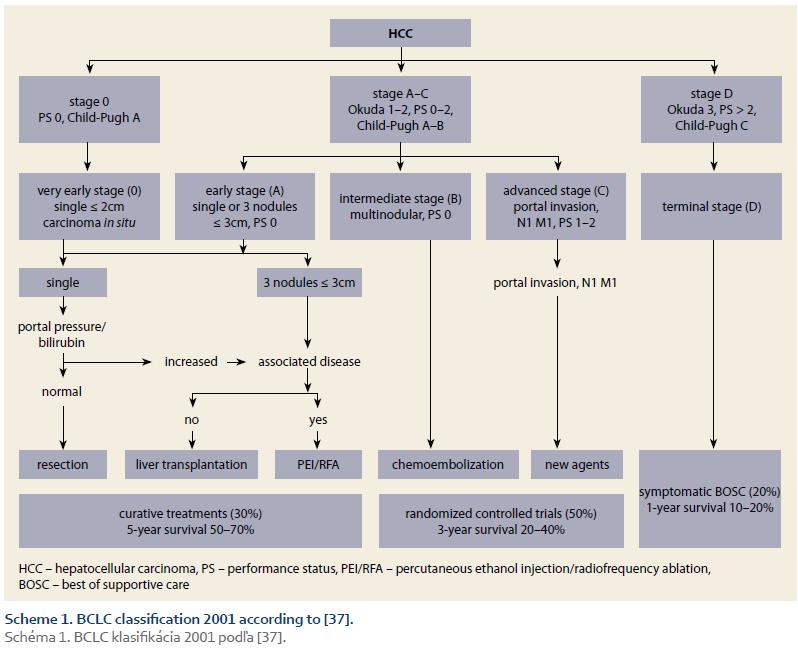
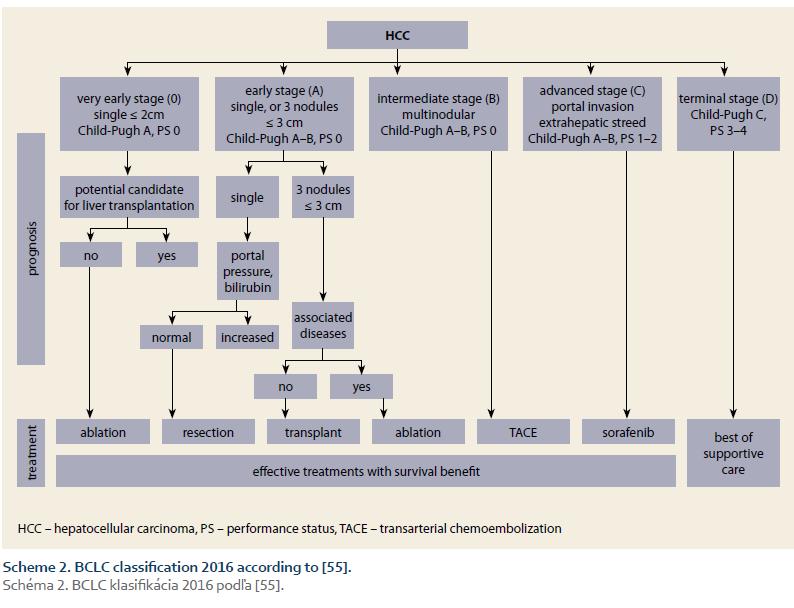
Prognostic classification (staging) is the main instrument of the effective management of HCC. Comparative studies showed that the best staging offers BCLC system (Scheme 1 – BCLC classification 2001; Scheme 2 – BCLC classification 2016) [37,55]. The main advantages of this system are that it takes into account all three domains decisive for prognosis (the tumour, the liver, and the patient), its prognostic accuracy across the stages, and the possibility to allocate treatment according to the stage [37,55–62].
Therapy
The so-called curative therapeutic modalities (RFA, SR, LTx) defined by the > 50% possibility of 5-year survival, can be used only in early-stage HCC (BCLC A); however, patients in this stage represent less than one third of all cases [42,61–63]. The other two thirds are divided to three groups with different recommended treatment approaches: 1. intermediate stage HCC – BCLC stage B (median survival 17–37 months), with recommended treatment by one of locoregional (liver-directed) modalities; 2. advanced stage – C (median survival 5.5 months) [64], treated by sorafenib; and 3. terminal stage – D (median survival 3 months), best of supportive care (BOSC) [65–69]. These simple-to-use allocation rules are not rigid due to the possibility of implementing a stage-migration strategy and an imperative of HCC management – multidisciplinary team discussions [70–73].
The aims of the study
The aims of the study are the following: 1. To determine the demographic and clinical characteristics of patients with HCC in a single-centre cohort; 2. to determine the proportion and characteristics of diagnosis of cases by the SUR-HCC; 3. to determine the distribution of BCLC stages at the time of diagnosis; 4. to determine allocation of therapy and 5. to determine prognosis in respect to the BCLC classes.
Patients and methods
In this retrospective study the analysis proceeded according to the following steps: The search of the Hospital Information System „Care Center®“ for the key Diagnosis „HCC“ (SAS). Retrieved med-ical records were subsequently compar-ed to the database of The Liver Unit and scrutinized for details with members of MDT. Patients seen between July 2007 and November 2016 were analysed.
1. Inclusion criterion – diagnosis of HCC according to the guidelines operating during the study interval [7,36–39,59].
2. Exclusion criterion – impossibility to retrieve sufficient data for final analysis.
3. Recorded variables – age, gender, presence/absence of liver cirrhosis as a stage of ACLD, etiology of ACLD, the means of diagnosis (by means of SUR-HCC, or otherwise), BCLC stage at diagnosis, diameter of lesions at diagnosis, treatment modality, survival.
4. Definitions – ACLD was diagnosed according to the accepted guidelines [59]; SUR-HCC was defined as the finding of at least two negative USG examinations 6-months apart, before determination of the HCC Diagnosis; the BCLC stages were determined using two consecutive sets of BCLC criteria (Scheme 1 and 2).
5. Treatment – the management was decided by the MDT according to allocation principles based on the BCLC system. The therapeutic armamentarium at the Liver Unit consisted of all the modalities endorsed by the Guidelines of the study interval, including LTx.
Results
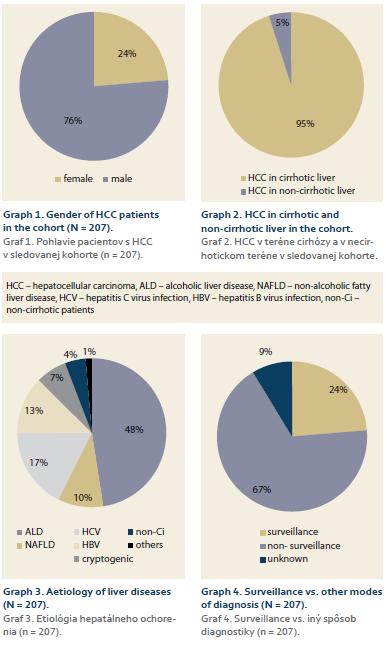
Cohort consisted of 207 patients, 197 with liver cirrhosis (95%), 158 men (76%), with median age of 65.9 (25–94) years (men vs. women = non-significant) (Graph 1 and 2).
Etiology
Etiology of underlying ACLD (and supposedly also the main etiology behind HCC): ALD – 106 patients (48%), HCV – 39 (17%), HBV – 28 (13%), NASH – 22 (10%), cryptogenic – 15 (7%), without cirrhosis – 10 (4%), others (primary biliary cholangitis, morbus Wilson, hereditary hemochromatosis) – 3 (1%) (Graph 3). In 49 cases (24%) the HCC was diagnosed by SUR-HCC, in 67% by other way, in remaining 9% the way of diagnosis was unknown (Graph 4). The average diameter of lesions at the time of diagnosis in SUR-HCC subgroup was 5.05 (0.75–20) cm, as compared to 8.6 (1–27.9) cm in patients without SUR-HCC (p = 0.001).
BCLC stages at admission
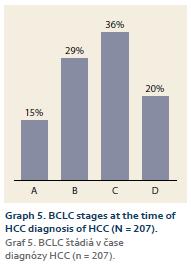
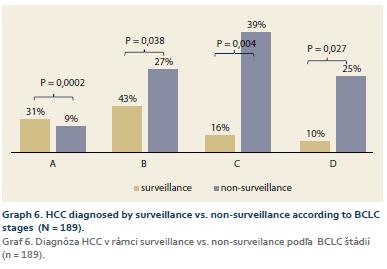
1. the whole cohort: BCLC A – 30 patients (15%), B – 61 patients (29%), C – 74 patients (36%), D – 42 patients (20%) (Graph 5); 2. according to the diagnosis modality (SUR-HCC vs. otherwise): A – 31% (15/49 patients) vs. 9% (12/140 patients) (p = 0.0002); B – 43% (21/49 patients) vs. 27% (38/140 patients) (p = 0.038); C – 16% (8/49 patients) vs. 39% (55/140 patients) (p = 0.004); D – 10% (5/49 patients) vs. 25% (35/140 patients) (p = 0.027) (Graph 6).
Treatment
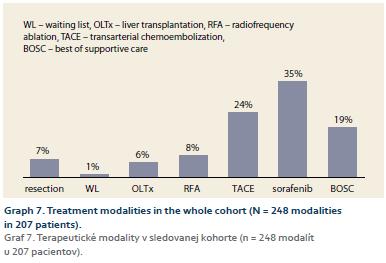
RFA 20 patients (8%), SR 17 patients (7%); LTx 14 patients (6%); DEB-TACE 60 patients (24%); sorafenib 88 patients (35%); BOSC 46 patients (19%), 3 patients were awaiting LTx (Graph 7).
Survival
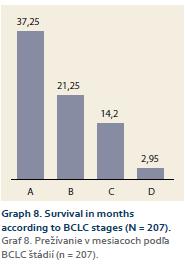
Mean survival in 1. the whole cohort was 17,34 (0,06–111.6) months; 2. according to BCLC stages: A – 37.25 (2.2–111.6) months; B – 21.25 (0.56–85.6) months; C – 14.2 (0.06–91.9) months; D – 2.95 (0.06–23.8) months (Graph 8).
Discussion
This analysis has several limitations in its retrospective and single-centre design, limited number of patients, and impossibility of subdividing early stage HCC to BCLC 0 (very early) and BCLC A. These drawbacks do not preclude it to be a reflection of characteristics and management of the HCC in this Central European region. Male predominance and the age of patients are comparable to other cohorts from Western Europe and North America, apart from the lack of age difference between genders; whether it is the signal of relevance will be seen over time [74,75].
As for the risk factors, liver cirrhosis was present in 95% of patients which falls well into the range of 98.3–56% found in the six studies from Germany, Netherlands, Italy, Denmark and Norway [76–79]. It is noticeable considering the trend of HCC to arise more and more in livers without cirrhosis (e. g. in 54% in the recent paper by Piscaglia) [32]. One possible explanation is an overestimation of the stage of ACLD in our cohort, since the liver biopsy was not a prerequisite for the diagnosis of cirrhosis; against this notion is the cumulative number of liver resecates in SR, liver explants in LTx, and hepatic venous pressure gradient measurements with no signal of over diagnosing the stage of ACLD. The other possibility is that the hit of the most renowned etiology of HCC without cirrhosis – NAFLD – has not yet been full-blown in this region [75,80]. Unfortunately, we were not able to ascertain the roles of the other common protective and risk factors – i.e. diet, smoking, obesity, type 2 diabetes mellitus (T2DM) and metabolic syndrome (MS). One can derive some information from their prevalence in the general population of Slovakia: Mediterran-ean diet (22.9%), smoking (41.2%), obesity (27.5%), T2DM (8.5%), and MS (10–84%) [81–85]. The etiology of underlying ACLD lends support to the “addictive” rather than “viral” pattern of the HCC in this region [86]. In nearly half the cases of HCC (48%), the main etiology was ALD. This is more than in several other studies from Europe (20–37%), but similar to the French prospective study [39,72,87,88]. The figure for ALD is considered to truly reflect the real-world influence of alcohol intake on health in Slovakia [89–94]. Another etiology attesting to the propensity of HCC in Slovakia to be of an “addictive” origin was the NAFLD/NASH. With 10% as the sole cause of LTx and another 40% as the possible co-factor, it can well be considered the second most important target for intervention. The roles of HCV and HBV, as well as the autoimmune syndromes were comparable to the figures from the other countries of this region [18,45,51,75]. Notably, none of the HCCs developed in the setting of HCV treatment with directly-acting antivirals.
Of concern is the low rate of diagno-sis by SUR-HCC (24%). Authors consider it to be a true reflection of the situation in Slovakia [95]. Comparable rates of SUR-HCC uptake seen in the literature is no reason for complacency [40,96–98]; on the contrary, it is taken as the cause of serious concern. In fact, this finding is considered to be the most important result of the analysis and the starting point for the optimization of SUR-HCC process in Slovakia. The second most important finding is the large diameter of the lesions detected by SUR-HCC (5.05 cm); although still significantly lower than that from diagnosis by other means (8.63 cm), it is also the cause of concern. It means that SUR-HCC was not only used at the suboptimal rate, but that it was also performed at the low level of quality.
Suboptimal quality notwithstanding, SUR-HCC was still associated with significantly less advanced BCLC stage at the diagnosis. The right-sided distribution of BCLC stages further illuminates the reserves of early diagnosis: only 15% of the cases fell into class A as compared to up to 42% elsewhere [99,100]. The results lend support to the value of SUR-HCC which has been supported by only one Asian randomized controlled trial (with several limitations) and therefore is still a matter of controversy: when we split our cohort according to diagnosis modality, the results have shown significantly more BCLC A + B cases with-, and significantly more C + D cases without SUR-HCC (Graph 3). Overall survival (17 months), and the survival according to the BCLC classes (37 months in BCLC A to 3 months in BCLC D) (Graph 4), were comparable to the results from the literature [99]. The possibility of curative treatment was low (RFA 8%, SR 7%, LTx 6%), which is probably the result of a trend to right-sided BCLC stage distribution at diagnosis. Even though the need for improvement is indisputable, a comparison of the results to those of El-Serag from the U.S. is somewhat relieving (RFA 4.1%, SR 8.2%, LTx 0.9%) [101]. Last but not least is the finding of the availability of all the recommended treatment modalities and their standard performance regarding survival.
Conclusions
1. Demographics in this cohort resemble those of HCC in the Western Europe and North America; 2. ALD was the most common etiology of ACLD; 3. Liver cirrhosis was diagnosed in 95% of the cases; 4. Only 24% of cases were detected via SUR-HCC; 5. Therefore the marked shift to the right in the BCLC stage distribution (A + B < C + D), leading to 6. Sub-optimal allocation of curative treatments and 7. Survival. According to these results, the management of HCC in our catchment area is full of reserves but still comparable to the standard of health care in Western Europe and North America.
Acknowledgement
We would like to thank Dr. Amit Singal an Assistant Professor of Internal Medicine for re-viewing the manuscript.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Submitted/Doručeno: 23. 10. 2017
Accepted/Přijato: 25. 11. 2017
Svetlana Adamcova-Selcanova, MD
HEGITO (Division of Hepatology, Gastroenterology and Liver Transplantation)
Department of Internal Medicine II Slovak Medical University
F. D. Roosevelt University Hospital
Nam. L. Svobodu 1
974 01 Banska Bystrica
Slovak Republic
sselcanova@gmail.com
Literature
1. Lozano R, Naghavi M, Foreman K et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380 (9859): 2095–2128. doi: 10.1016/S0140-6736 (12) 61728-0.
2. Jemal A, Simard EP, Dorell C et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV) -associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013; 105 (3): 175–201. doi: 10.1093/jnci/djs491.
3. Noureddin M, Rinella ME. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis 2015; 19 (2): 361–379. doi: 10.1016/j.cld.2015.01.012.
4. Archambeaud I, Auble H, Nahon P et al. Risk factors for hepatocellular carcinoma in Caucasian patients with non-viral cirrhosis: the importance of prior obesity. Liver Int 2015; 35 (7): 1872–1876. doi: 10.1111/liv.12767.
5. Omata M, Dan Y, Daniele B et al. Clinical features, etiology, and survival of hepatocellular carcinoma among different countries. J Gastroenterol Hepatol 2002; 17 (Suppl): S40–S49.
6. Bosch FX, Ribes J, Diaz M et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004; 127 (5 Suppl 1): S5–S16.
7. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology 2004; 127 (5 Suppl 1): S27–S34.
8. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132 (7): 2557–2576.
9. Khan SA, Taylor-Robinson SD, Toledano MB el al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 2002; 37 (6): 806–813.
10. De Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63 (3): 743–752. doi: 10.1016/j.jhep.2015.05.022.
11. Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol 2014; 28 (5): 753–770. doi: 10.1016/j.bpg.2014.08.007.
12. Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65 (2): 87–108. doi: 10.3322/caac.21262.
13. Bosch FX, Ribes J, Cléries R et al. Epidemiology of hepatocellular carcinoma. Clin Liver Dis 2005; 9 (2): 191–211.
14. World Health Organization, International Agency for Cancer Research. The global burden of disease. 2004 update. Globocan 2008. [online]. Available from: http: //www.who.int/healthinfo/global_burden_disease/GBD_report_2004 update_full.pdf.
15. Frey RS, Boldanova T. Heim MH. Ultrasound surveillance for hepatocellular carcinoma: real-life performance in a hepatology outpatient clinic. Swiss Med Wkly 2015; 145: w14200. doi: 10.4414/smw.2015.14200.
16. Harrison SA, Diehl AM. Fat and the liver: a molecular overview. Semin Gastrointest Dis 2002; 13 (1): 3–16.
17. Festi D, Colecchia A, Sacco T et al. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev 2004; 5 (1): 27–42.
18. Skladaný Ľ (ed). Infekcia vírusom hepatitídy B a hepatocelulárny karcinóm. Trendy v hepatol 2009; 3: 12–21.
19. Skladaný Ľ, Adamcová-Selčanová S, Badinková J et al. Hepatocelulárny karcinóm. Onkológia VIP 2013: 1–8.
20. Skladaný Ľ, Adamcová-Selčanová S, Pritzová E. Hepatocelulárny karcinóm BCLC-C liečený sorafenibom. Farmakoterapia 2013; 3: 20–25.
21. Adamcová-Selčanová S, Skladaný Ľ, Badinková J. Hepatocelulárny karcinóm a využitie sorafenibu. Farmakoterapie 2013; 3 (2): 78–80.
22. EL-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology 2014; 60 (5): 1767–1775. doi: 10.1002/hep.27222.
23. Njei B, Rotman Y, Ditah I et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015; 61 (1): 191–199. doi: 10.1002/hep.27388.
24. Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer 2015; 4 (1): 39–50. doi: 10.1159/000367727.
25. Sawyers CL, Abate-Shen C, Anderson KC et al. AACR cancer progress report 2013. Clin Cancer Res 2013; 19 (20 Suppl): S4–S98. doi: 10.1158/1078-0432.CCR-13-2107.
26. Benvegnù L, Gios M, Boccato S et al. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut 2004; 53 (5): 744–749.
27. Skladaný Ľ, Adamcová-Selčanová S, Dropčová A et al. Transplantácia pečene. Interná Med 2015; 3: 93–101.
28. Lang K, Danchenko N, Gondek K et al. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol 2009; 50 (1): 89–99. doi: 10.1016/j.jhep.2008.07.029.
29. Arguedas MR, Chen VK, Eloubeidi MA et al. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol 2003; 98 (3): 679–690.
30. Armstrong GL, Alter MJ, McQuillan GM et al. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000; 31 (3): 777–782.
31. Singal A, Volk ML, Waljee A et al. Metaanalysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009; 30 (1): 37–47. doi: 10.1111/j.1365-2036.2009.04014.x.
32. Piscaglia F, Svegliati-Baroni G, Barchetti A et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 2016; 63 (3): 827–838. doi: 10.1002/hep.28368.
33. Perumpail RB, Wong RJ, Ahmed A et al. Hepatocellular carcinoma in the setting of non-cirrhotic nonalcoholic fatty liver disease and the metabolic syndrome: US experience. Dig Dis Sci 2015; 60 (10): 3142–3148. doi: 10.1007/s10620-015-3821-7.
34. Farinati F, Sergio A, Baldan A et al. Early and very early hepatocellular carcinoma: when and how much do staging and choice of treatment really matter? A multi-center study. BMC Cancer 2009; 9: 33. doi: 10.1186/1471-2407-9-33.
35. Wang C, Wang H, Yang W et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015; 61: 1579–1590. doi: 10.1002/hep.27548.
36. Žigrai M, Skladaný Ľ, Hrušovský Š et al. Štandardný diagnostický a terapeutický postup. Manažment liečby hepatocelulárneho karcinómu. Metodický list racionálnej farmakoterapie 2013; 16: 1–8.
37. Bruix J, Sherman M, Llovet JM et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35 (3): 421–430.
38. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53 (3): 1020–1022. doi: 10.1002/hep.24199.
39. European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56 (4): 908–943. doi: 10.1016/j.jhep.2011.12.001.
40. Bolondi L, Sofia S, Siringo S et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost-effectiveness analysis. Gut 2001; 48 (2): 251–259.
41. Sherman M, Bruix J, Porayko M et al. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology 2012; 56 (3): 793–796. doi: 10.1002/hep.25869.
42. Altekruse SF, McGlynn KA, Reichman ME et al. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27 (9): 1485–1491. doi: 10.1200/JCO.2008.20.7753.
43. Mittal S, Sada YH, El-Serag HB et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol 2015; 13 (3): 594–601. doi: 10.1016/j.cgh.2014.08. 013.
44. Sangiovanni A, Del Ninno E, Fasani P. Increas-ed survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004; 126 (4): 1005–1014.
45. Colombo M. Natural history and pathogenesis of hepatitis C virus related hepatocellular carcinoma. J Hepatol 1999; 31 (Suppl 1): 25–30.
46. Velázquez RF, Rodríguez M, Navascués CA et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology 2003; 37 (3): 520–527.
47. Pateron D, Ganne N, Trinchet JC et al. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol 1994; 20 (1): 65–71.
48. Chan EY, Larson AM, Fix OK et al. Identifying risk for recurrent hepatocellular carcinoma after liver transplantation: implications for surveillance studies and new adjuvant therapies. Liver Transpl 2008; 14 (7): 956–965. doi: 10.1002/lt.21449.
49. Yuen MF, Cheng CC, Lauder IJ et al. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology 2000; 31 (2): 330–335.
50. Toyoda H, Kumada T, Osaki Y el al. Staging hepatocellular carcinoma by a novel scoring system (balad score) based on serum markers. Clin Gastroenterol Hepatol 2006; 4 (12): 1528–1536.
51. Mc Mahon BJ, Bulkow L, Harpster A el al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology 2000; 32 (4 Pt 1): 842–846.
52. Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin Mol Hepatol 2016; 22 (1): 7–17. doi: 10.3350/cmh.2016.22.1.7.
53. Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB (Oxford) 2005; 7 (1): 26–34. doi: 10.1080/13651820410024 049.
54. Bialecki ES, Ezenekwe AM, Brunt EM et al. Comparison of liver biopsy and noninvasive methods for diagnosis of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2006; 4 (3): 361–368.
55. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016; 150 (4): 835–853. doi: 10.1053/j.gastro.2015.12.041.
56. Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002; 35 (3): 519–524.
57. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42 (5): 1208–1236.
58. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53 (3): 1020–1022. doi: 10.1002/hep.24199.
59. Forner A, Hessheimer AJ, Isabel Real M et al. Treatment of hepatocellular carcinoma. Crit Rev Oncol Hematol 2006; 60 (2): 89–98.
60. Oliverius M, Bĕlina F, Novotný J et al. Treatment of hepatocellular carcinoma and current situation in the Czech Republic. Rozhl Chir 2007; 86 (12): 635–641.
61. Forner A, Reig ME, De Lope CR et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010; 30 (1): 61–74. doi: 10.1055/s-0030-1247133.
62. Brůha R, Drastich P, Hůlek P et al. Diagnostika a léčba hepatocelulárního karcinomu. Vnitr Lek 2005; 51 (12): 1406–1408.
63. Mazzaferro V, Llovet JM, Miceli R et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009; 10 (1): 35–43. doi: 10.1016/S1470-2045 (08) 70284-5.
64. Suchánková G, Špičák J. Sorafenib in the treatment of hepatocellular carcinoma. Vnitr Lek 2011; 57 (5): 485–490.
65. Gomaa AI, Waked I. Recent advances in multidisciplinary management of hepatocellular carcinoma. World J Hepatol 2015; 7 (4): 673–687. doi: 10.4254/wjh.v7.i4.673.
66. Bruix J, Llovet JM, Castells A et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998; 27 (6): 1578–1583.
67. Llovet JM, Real MI, Montana X et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359 (9319): 1734–1739.
68. Marelli L, Stigliano R, Triantos C et al. Treatment outcomes for hepatocellular carcinoma using chemoembolization in combination with other therapies. Cancer Treat Rev 2006; 32 (8): 594–606.
69. Varela M, Real MI, Burrel M et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007; 46 (3): 474–481.
70. Abou-Alfa GK, Schwartz L, Ricci S et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006; 24 (26): 4293–4300.
71. Kim Je, Ryoo BY, Ryu MH et al. Sorafenib for hepatocellular carcinoma according to Child-Pugh class of liver function. Cancer Chemother Pharmacol 2011; 68 (5): 1285–1290. doi: 10.1007/s00280-011-1616-x.
72. Llovet J, Ricci S, Mazzaferro V et al. Randomized phase III trial of sorafenib versus placebo in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2007; 25 (Suppl 18): Abstract LBA1.
73. Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? Oncologist 2006; 11 (7): 790–800.
74. Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136 (5): E359–E386. doi: 10.1002/ijc.29210.
75. Park JW, Chen M, Colombo M et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015; 35 (9): 2155–2166. doi: 10.1111/liv.12818.
76. Giannini EG, Cucchetti A, Erroi V et al. Surveillance for early diagnosis of hepatocellular carcinoma: How best to do it? World J Gastroenterol 2013; 19 (47): 8808–8821. doi: 10.3748/wjg.v19.i47.8808.
77. Weinmann A, Koch S, Niederle IM et al.Trends in epidemiology, treatment, and survival of hepatocellular carcinoma patients between 1998 and 2009: an analysis of 1066 cases of a German HCC Registry. J Clin Gastroenterol 2014; 48 (3): 279–289. doi: 10.1097/MCG.0b013e3182a8a793.
78. Jepsen P, Andersen MW, Villadsen GE et al. Time-trends in incidence and prognosis of hepatocellular carcinoma in Denmark: a nationwide register-based cohort study. Liver Int 2017; 37 (6): 871–878. doi: 10.1111/liv.13340.
79. Eskesen AN, Bjøro K, Aandahl EM et al. Low use of surveillance and early diagnosis of hepatocellular carcinoma in Norway – a population-based cohort study. Cancer Epidemiol 2014; 38 (6): 741–747.
80. Duan XY, Qiao L, Fan JG. Clinical features of nonalcoholic fatty liver disease-associated hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2012; 11 (1): 18–27.
81. Leung CH, Yeoh SW, Patrick D et al. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol 2015; 21 (4): 1189–1196. doi: 10.3748/wjg.v21.i4.1189.
82. Ng M, Freeman MK, Fleming TD el al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 2014; 311 (2): 183–192. doi: 10.1001/jama.2013.284692.
83. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016; 387 (10027): 1513–1530. doi: 10.1016/ S0140-6736 (16) 00618-8.
84. Hoşcan Y, Yiğit F, Müderrisoğlu H. Adherence to Mediterranean diet and its relation with cardiovascular diseases in Turkish population. Int J Clin Exp Med 2015; 8 (2): 2860–2866.
85. Turati F, Trichopoulos D, Polesel J et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol 2014; 60 (3): 606–611. doi: 10.1016/j.jhep.2013.10.034.
86. Pisani P, Parkin DM, Muñoz N et al. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev 1997; 6 (6): 387–400.
87. Bucci L, Garuti F, Lenzi B et al. The evolutionary scenario of hepatocellular carcinoma in Italy: an update. Liver Int 2017; 37 (2): 259–270. doi: 10.1111/liv.13204.
88. Dyson J, Jaques B, Chattopadyhay D et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol 2014; 60 (1): 110–117. doi: 10.1016/j.jhep.2013.08.011.
89. Szántová M. Alkohol a pečeň pohľadom hepatológa. Psychiatr prax 2013; 14 (3): 114–119.
90. Szántová M. Epidemiologický prehľad spotreby alkoholu v SR a ČR. Prakt Lék 2006; 86 (7): 374–380.
91. Szántová M. Diagnostika a liečba alkoholového poškodenia pečene. Via pract 2008; 5 (4–5): 170–172.
92. Szántová M. Alkoholová choroba pečene vo svetle nových EASL odporúčaní. Intern Med 2012; 12 (9): 433–440.
93. Szántová M. Alkoholová choroba pečene. Alkoholizmus a drogové závislosti (protialkoholický obzor) 2013; 48 (1): 25–35.
94. Rehm J, Shield KD, Rehm M et al. Alcohol consumption, alcohol dependence and attributable burden of disease in Europe: potential gains from eff ective interventions for alcohol dependence. Toronto: CAMH 2012.
95. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996; 24 (2): 289–293.
96. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130 (7): 417–422.
97. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United states of America. J Gastroenterol Hepatol 2001; 16 (5): 553–559.
98. Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol 2015; 13 (12): 2140–2151. doi: 10.1016/j.cgh.2015.08.014.
99. Gomaa AI, Hashim MS, Waked I. Comparing staging systems for predicting prognosis and survival in patients with hepatocellular carcinoma in Egypt. PLoS One 2014; 9 (3): e90929. doi: 10.1371/journal.pone.0090929.
100. Fenoglio L, Serraino C, Castagna E et al. Epidemiology, clinical-treatment patterns and outcome in 256 hepatocellular carcinoma cases. World J Gastroenterol 2013; 19 (21): 3207–3216. doi: 10.3748/wjg.v19.i21.3207.
101. El-Serag HB et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol 2006; 44 (1): 158–166.