Late tension pneumoperitoneum via the abdominal stoma after PEG-J
Martina Jakabovičová1, Xénia Faktorová1, Mária Szantová1, Zuzana Ďurkovičová1
+ Affiliation
Summary
A 71-year-old woman with Parkinson’s disease underwent elective percutaneous endoscopic gastrojejunostomy due to levodopa-carbidopa intestinal gel treatment. Less than one week after the procedure, the patient developed abdominal distension. X-ray and computed tomography revealed massive pneumoperitoneum. An external binder was dislocated above the abdominal wall. After replacing the binder and fixating it onto the skin, imaging showed no regression of free air. Drainage was performed using a Redon drain, and a gush of free air and ascites leaked out of the abdominal wall. Ventilatory air leakage beside the gastric tube caused permanent massive pneumoperitoneum. After the removal of the PEG tube, the patient fully recovered. In this article, we present a late complication of pneumoperitoneum with air leakage from the abdominal wall stoma. This complication is rarer than air leakage from the visceral wall stoma.
Keywords
pneumoperitoneum, percutaneous endoscopic gastrostomy, free air
Clinical and experimental gastroenterology: case report
Introduction
Percutaneous endoscopic gastrostomy (PEG) is currently the most favorite procedure, providing long-term enteral nutrition, and has almost completely replaced surgical gastrostomy [1]. Percutaneous endoscopic gastrojejunostomy (PEG-J) is a modality developed for patients with gastric motility disorders to provide enteral access for nutrition or medical treatment that bypasses the stomach. Although PEG is considered a safe procedure, tube placement is associated with potential complications [2]. In most cases, pneumoperitoneum is benign and resolves spontaneously, while complicated pneumoperitoneum, such as bowel injury, rarely occurs [3]. The incidence of pneumoperitoneum after a tube placement varies from 4.7% to 55.6%, before the fistula path is created. Fistula path creation takes approximately 14–20 days [4]. Pneumoperitoneum usually occurs 3 h after PEG insertion and is determined spontaneously within a week [5]. Asymptomatic and stable patients are kept under surveillance, and usually no intervention is needed. In patients with significant signs of complicated pneumoperitoneum, such as abdominal pain or distension, fever, hemodynamic instability, leukocytosis, free fluid, or massive free air in the abdominal cavity, surgical intervention should be taken into consideration [6]. Herein, we describe a patient with massive and permanent pneumoperitoneum and peritonitis that developed less than one week after PEG-J insertion.
Case report
A 71-year-old female with arterial hypertension and hyperlipidemia was referred to the endoscopic unit for elective percutaneous endoscopic gastrojejunostomy with an extension due to Parkinson’s disease treatment. We performed PEG using the “pull” technique introduced by Gauderer et al. Esophagogastroduodenography was performed after the patient fasted without sedation. After gastric insufflation and gastric content aspiration abdominal wall transillumination was performed, and the correct location for PEG insertion was identified. Local anesthesia was applied, and PEG was inserted using a sterile technique. A 15 Fr PEG tube was inserted, and a 9 Fr jejunal tube was placed via a PEG tube behind the ligament of Treitz using endoscopic forceps. The procedure was uneventful, without periprocedural complications. A prophylactic dose of broad-spectrum antibiotics was administered. We started a levodopa-carbidopa intestinal gel therapy 1 hour after PEG-J insertion and peroral feeding the next day after 24 hours of fasting without any complications. On the sixth day of her inpatient stay, the patient’s abdomen was distended without tenderness, and the patient did not complain of any pain. In addition to abdominal enlargement, a peristomal leak with a dislocation of an external bumper 1 cm above the abdominal wall was noticed. Gastroscopy confirmed a normal internal bumper and gastric tube position. We performed a thoraco-abdominal X-ray, and free air was present (Fig. 1A). The patient’s temperature was normal, and she was hemodynamically stable throughout inpatient care. A full blood count demonstrated a white blood cell (WBC) count of 10.7 × 109/l with normal differential, hemoglobin and platelet (PLT) levels. Further laboratory analyses revealed the following results: C-reactive protein (CRP) 247.2 mg/l, procalcitonin 0.18 μg/l, and no other signs of sepsis. Urine and stool examinations were normal. Her albumin level was lower than normal (28.3 g/l). We replaced the external bumper and fixed it with wound dressing. The patient was left nil per mouth, and artificial nutrition supply, human albumin, and antimicrobial therapy were given. Antiparkinson therapy was given subcutaneously. On day 10 after PEG-J insertion, a second dislodgement of the external bumper was observed (Fig. 2A). An abdominal X-ray and a computed tomography (CT) scan revealed tension pneumoperitoneum in progression with ascites and starting anasarca (Fig. 1B, 2). The gastric tube was dislocated but markedly inside the stomach (Fig. 2B). Laboratory tests showed an increase in inflammatory markers. A blood count demonstrated an increase in the WBC count to 13.7 × 109/l with neutrophilia (11.8 × 109/l), a PLT count of 537 × 1012/l, and a CRP level of 334.2 mg/l. Percutaneous air drainage was performed with insertion of a Redon drain (Ch 12) into the right hypochondriac region, and a gush of free air and clear ascites leaked out of the abdominal wall. Despite our intervention and repeated replacement of an external bumper to its correct position, free air repeatedly aspirated into the peritoneal cavity, which confirmed the patient’s acoustic sensation of air aspiration beside the PEG tube, and peritoneal drainage – was not sufficient. We were convinced that the patient had “ventilator” air influx through the external stoma site. We withdrew the PEG-J tube on the fourteenth day after insertion and continued with antimicrobial therapy. The Redon drain was withdrawn five days later. We planned new PEG reinsertion at a different site, but on the patient’s request, we cancelled it. Nine days after the PEG device was removed, the patient fully recovered (Fig. 1C), and a surgical approach was not needed.
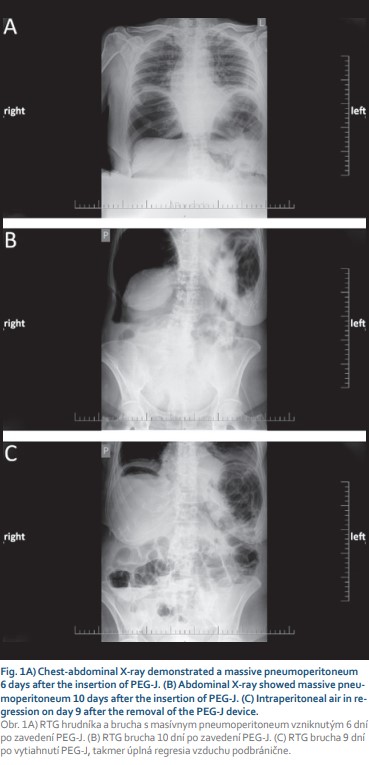
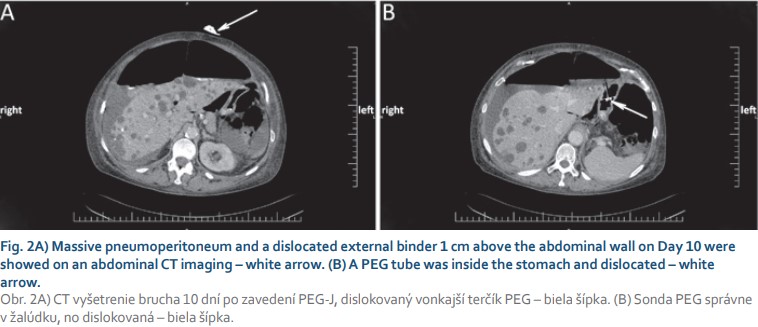
Discussion
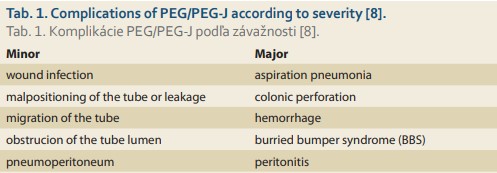
PEG is a frequently used and favored endoscopic procedure to provide enteral feeding when oral intake is inadequate or not possible [7]. PEG-J tube insertion is a modality designated for long-term jejunal nutrition or the administration of medicine to the stomach. Several approaches have been developed, such as endoscopic, radiological, and open approaches. We performed PEG with jejunal extension. Complications of PEG are infrequent, and vary in severity; thus, they are divided into two groups, as summarized in Tab. 1 [8].
Pneumoperitoneum is usually benign in nature and resolves spontaneously [9]. Its incidence is relatively frequent, although pneumoperitoneum is generally considered a minor complication and may occur within 3 h after PEG tube placement [10]. Post-PEG pneumoperitoneum may be related to the high intragastric air pressure produced by air insufflation of the endoscope in association with needle puncture of the abdominal wall and stomach [11]. Most cases typically resolve within a week. Pneumoperitoneum is a clinical concern, only if it is found in the presence of peritonitis, an inflammatory response, or sepsis or with a worsening amount of intraperitoneal air [12]. Some important factors affect air absorption, such as difficult wound healing due to malnutrition, diabetes, immunosuppressive treatment, and the amount of air initially present.
In patients with neurodegenerative diseases, the rate of complications is high. These patients are at increased risk of morbidity, aspiration, infection, and a poor nutritional status. Other known prognostic factors that influence PEG outcomes are a low serum cholesterol level, a low lymphocyte count, and comorbidities [13]. Pih et al confirmed a high incidence of pneumoperitoneum in a group of patients with neurodegenerative diseases but the severity of pneumoperitoneum was not related to the neurological diseases [14].
In the present case, there were no complications for up to six days. Pneumoperitoneum occurred as a late complication. We believe ventilatory pneumoperitoneum developed as a result of air influx into the peritoneal cavity beside the PEG tube, which confirms the patient’s acoustic sensation of air aspiration into the abdominal cavity and the progression of pneumoperitoneum. We suppose that insufficient gastric tube fixation caused remarkable air leakage, developing an “air valve”, where the abdominal wall was the air leakage site. In contrast, visceral site stoma air leakage is an early complication.
Parkinson’s disease, type 2 diabetes and malnutrition are risk factors for pneumoperitoneum and peritonitis development and are associated with poor wound healing and pneumoperitoneum severity. As one rules out intestinal perforation and air leakage at a visceral site, air leakage through the abdominal stoma site is one possible causes of pneumoperitoneum after PEG insertion [15]. To our knowledge, our case is the second of massive tension pneumoperitoneum occurring due to air influx through the external PEG stoma site [15].
It is unclear how to prevent this complication, but thorough technical PEG performance and faultless materials, equipment and facilities are necessary.
Submitted/Doručené: 13. 12. 2020
Accepted/Prijaté: 24. 3. 2020
Zuzana Ďurkovičová, MD
3rd Department of Internal Medicine
University Hospital of Comenius University in Bratislava
Limbova 5
833 05 Bratislava
Slovakia
zuz.sedlakova@gmail.com
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Ponsky JL. The development of PEG: how it was. J Interv Gastroenterol 2011; 1(2): 88–89. doi: 10.4161/jig.1.2.16831.
2. Lloyd DA, Powell-Tuck J. Artificial nutrition: principles and practice of enteral feeding. Clin Colon Rectal Surg 2004; 17(2): 107–118. doi: 10.1055/s-2004-828657.
3. Milanchi S, Allins A. Early pneumoperitoneum after percutaneous endoscopic gastrostomy in intensive care patients: sign of possible bowel injury. Am J Crit Care 2007; 16(2): 132–136.
4. Lee JY, Park KS. Pneumoperitoneum after percutaneous endoscopic gastrostomy: does it have clinical significance? Intest Res 2015; 13(4): 295–296. doi: 10.5217/ir.2015.13.4.295.
5. Varela Sánchez Z, Pareja Ciuró F, Docobo Durántez F et al. Giant pneumoperitoneum secondary to percutaneous endoscopic gastrostomy. Rev Esp Enferm Dig 2007; 99(2): 121–122.
6. Barbeiro S, Martins C, Goncalves C. Pneumoperitoneum after percutaneous endoscopic gastrostomy. GE Port J Gastroenterol 2015; 22(2): 75–76. doi: 10.1016/j.jpge.2015.01.004.
7. Kohout P, Skladaný Ľ. Perkutánní endoskopická gastrostomie. In: Kohout P, Skladaný Ľ (eds). Perkutánní endoskopická gastrostomie a její místo v algoritmu umělé výživy. 1. vyd. Praha: Galén 2002: 69–79.
8. Rahnemai-Azar AA, Rahnemaiazar AA, Naghshizadian R et al. Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World J Gastroenterol 2014; 20(24): 7739–7751. doi: 10.3748/wjg.v20.i24.7739.
9. Kroupa R, Kohout P, Cyrany J et al. Percutaneous endoscopic gastrostomy – Czech Society of Gastroenterology Guidelines. Gastroent Hepatol 2019; 73(3): 195–207. doi: 10.14735/amgh2019195.
10. Gottfried EB, Plumser AB, Clair MR. Pneumoperitoneum following percutaneous endoscopic gastrostomy. A prospective study. Gastrointest Endosc 1986; 32(6): 397–399. doi: 10.1016/s0016-5107(86)71919-6.
11. Park WY, Lee TH, Lee JS et al. Reappraisal of pneumoperitoneum after percutaneous endoscopic gastrostomy. Intest Res 2015; 13(4): 313–317. doi: 10.5217/ir.2015.13.4.313.
12. Alley JB, Corneille MG, Stewart RM et al. Pneumoperitoneum after percutaneous endoscopic gastrostomy in patients in the intensive care unit. Am Surg 2007; 73(8): 765–768.
13. Sarkar P, Cole A, Scolding NJ et al. Percutaneous endoscopic gastrostomy tube insertion in neurodegenerative disease: a retrospective study and literature review. Clin Endosc 2017; 50(3): 270–278. doi: 10.5946/ce.2016.106.
14. Pih GY, Na HK, Ahn JY et al. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy insertion. BMC Gastroenterol 2018; 18(1): 101. doi: 10.1186/s12876-018-0825-8.
15. Vijayakrishnan R, Adhikari D, Anand CP. Recurrent tense pneumoperitoneum due to air influx via abdominal wall stoma of a PEG tube. World J Radiol 2010; 2(7): 280–282. doi: 10.4329/wjr.v2.i7.280.