Liver function test abnormality in paediatric patients with Covid-19 – a single-centre study in the south of Iran
Amir Saeed1, Amir Naghshzan2, Seyed Mohsen Dehghani3
+ Affiliation
Summary
Introduction: From the beginning of the Covid-19 outbreak, liver involvement has been reported in some adult studies and some possible mechanisms have been proposed for this. However, there are few studies in the paediatric age group. In this study, we aimed to determine the incidence rate of liver damage in paediatric patients with Covid-19 and their impact on the outcome. Methods: All Covid-19 RT-PCR positive children admitted to the Namazi Hospital in Shiraz, Iran, from March 2020 to April 2021 were enrolled. Clinical data, especially gastrointestinal signs and symptoms, and paraclinical data, including liver function tests, C-reactive protein (CRP) and lactate dehydrogenase (LDH) were gathered and all the patients were monitored during their hospital stay. Results: One hundred and ten patients were enrolled in this study and of them 67 patients (69%) were admitted to the PICU. Gastrointestinal symptoms were seen in 47 patients (42.7%) upon admission. Abnormally high AST and ALT were seen in 83 (75.4%) and 49 (44.5%) patients, respectively. In the patients admitted to the PICU (n = 67), 56 (83.6%) had abnormal AST and 34 (50.7%) had high ALT. Twenty-seven (62.8%) and 15 (34.9%) of the 43 patients who were not admitted to the PICU had high AST and ALT, respectively. The patients admitted to the PICU had significantly higher CRP and LDH and lower albumin levels. Logistic Regression analysis revealed that CRP, LDH and albumin are predictors of mortality. Conclusion: Abnormal liver function tests in children with Covid-19 are common and we can consider hypoalbuminaemia in addition to high LDH and CRP as poor prognostic factors.
Keywords
prognosis, liver function tests, COVID-19, liver damage
Introduction
It was in December 2019 when an outbreak of respiratory symptoms and pneumonia were reported from Wuhan in China and, from there, soon spread to other countries. In February 2020, the World Health Organization (WHO) declared it a pandemic. In our country, Iran, it was March 2020 when the first cases were detected. In the first articles, the incidence rate, severity and mortality in paediatric cases were low. However, soon after, several reports appeared on severe cases of paediatric patients with Covid-19 and extra-pulmonary manifestations of Covid-19.
There are three types of coronaviruses that cause disease in humans; severe acute respiratory syndrome coronavirus (SARS-CoV), the 2019 coronavirus (SARS-CoV-2), and the Middle East respiratory syndrome coronavirus (MERS-CoV) [1]. All three viruses can cause extra-pulmonary manifestations such as liver, cardiac, kidney and neuronal diseases [2].
Some studies have shown liver damage in patients with these three viruses in different degrees [3–5]. Some possible mechanisms for liver damage during infection with coronaviruses were suggested, including direct invasion of the liver [6,7], systemic inflammatory response to SARS-CoV infection, hypoxia and side effects of drugs used for the treatment of these patients [8–10].
To date, some limited studies investigate liver damage among paediatric patients infected with SARS-CoV-2 [11–13], so we conducted this study to determine the incidence rate of liver damage in paediatric patients with Covid-19 and its outcome in our centre.
Material and Methods
All children less than 18 years of age with positive Covid-19 RT-PCR (reverse transcriptase-polymerase-chain-reaction assay of a specimen collected on an oropharyngeal swab, nasopharyngeal swab or bronchoalveolar lavage) who were admitted to the paediatric ward of Namazi Hospital in Shiraz, Iran from March 2020 to April 2021 were enrolled and those who did not have liver function tests were excluded from the study. Demographics, gastrointestinal signs and symptoms and laboratory data, including liver function tests (aspartate aminotransferase: AST; alanine aminotransferase: ALT; total and direct bilirubin; and albumin), C-reactive protein (CRP) and lactate dehydrogenase (LDH) were collected. All the patients were monitored during their hospital stay. The data were analysed with IBM SPSS Statistics for Windows, Version 23.0 (IBM Co., Armonk, NY, USA). Continuous variables were calculated as means ± standard deviations (SDs), whereas categorical variables were expressed as numbers and percentages. Comparisons between the groups were analysed using the independent t-test and Mann-Whitney test for continuous variables and the Chi-square test for categorical variables. Logistic regression analysis was used for estimating odds ratios (ORs) and confidence intervals (CIs) to evaluate the impact of various variables on outcome. A P-value less than 0.05 was considered statistically significant.
Results
Of the one hundred and seventy-nine Covid-19 PCR positive patients admitted to our centre, 110 cases had liver function tests and were enrolled in the study. 57 out of 110 patients (51.8%) were boys and 67 patients (69%) were admitted to the PICU. Thirty-one patients had chronic disease and of them, ten patients had known liver disease; in our study 14 patients died due to respiratory failure and septic shock and of these, nine patients had chronic disease (one of them had chronic liver disease and his death was due to respiratory failure). Gastrointestinal symptoms were seen in 47 patients (42.7%) at admission; abdominal pain in 28 (25.4%), nausea and vomiting in 11 (10%) and diarrhoea in 8 (7.3%) cases.
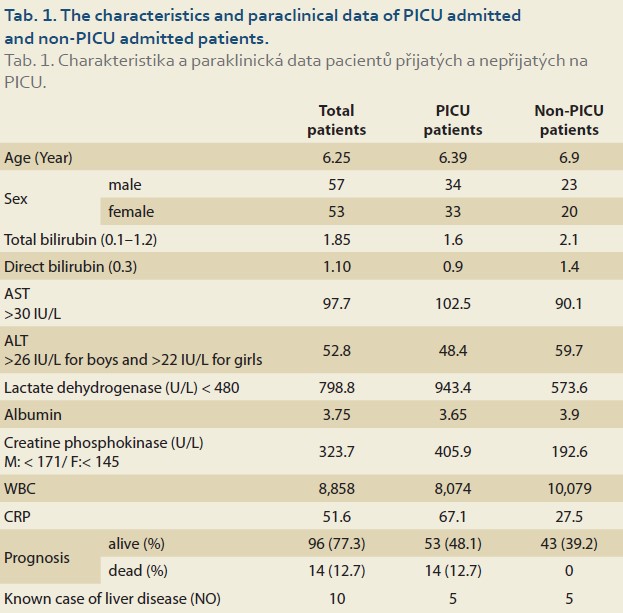
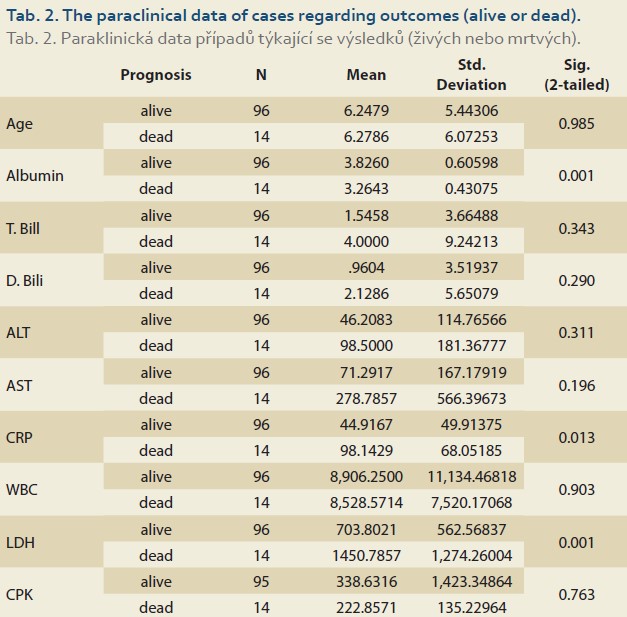
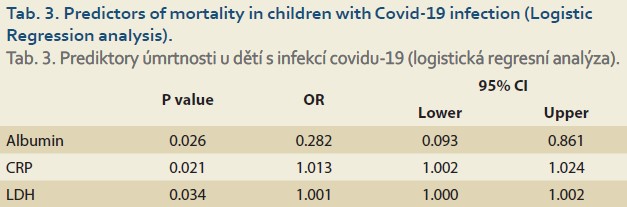
Of 110 patients, 83 patients (75.4%) had abnormally high AST and 49 (44.5%) had high ALT (>26 IU/L for boys and >22 IU/L for girls), and of the patients admitted to the PICU (67 cases) 56 (83.6%) had abnormal AST and 34 (50.7%) had high ALT. 27 (62.8%) and 15 (34.9%) of 43 patients who were not admitted to the PICU had high AST and ALT, respectively. The patients admitted to the PICU had statistically significantly higher abnormal AST than those not admitted to the PICU (OR = 3.017; 95% CI 1.233–7.380; p = 0.013), but this difference was not significant regarding ALT (OR = 1.923; 95% CI 0.874–4.234; p = 0.102). Table 1 shows the characteristics and paraclinical data of the patients admitted to the PICU and those not admitted to the PICU. The patients admitted to the PICU had significantly higher CRP and LDH and lower albumin levels. Abnormally high bilirubin and hypoalbuminaemia were seen in 26 (23.6%) and 37 (33.6%) patients, respectively. Elevated CRP was seen in 75 (68.2%) patients and there was no correlation between high CRP and AST (r = 0.170; p = 0.075) or ALT (r = 0.126; p = 0.189) levels. In our study, the CPK level was high; especially in patients who were admitted to the PICU and of them none was due to muscular origin, but 16 patients who were excluded from the list had an elevated troponin level (Table 1). The total mortality rate was 12.7% and all were admitted to the PICU. The para- clinical data of cases regarding outcomes (alive or dead) are presented in Table 2. Elevated CRP [SE = 14.99; 95% CI –82.96– (–23.49) ]; p = 0.013) and LDH [SE = 196.93; 95% CI –1137.33– (–356.63); p = 0.001] and low albumin levels (SE = 0.168; 95% CI 0.23–0.89; p = 0.001) were significantly associated with mortality. Logistic Regression analysis revealed that CRP, LDH and albumin are predictors of mortality (Table 3).
Discussion
Some possible mechanisms are considered for the increase of liver enzymes in patients infected with Covid-19 and those with liver damage. The viral infection itself, pre-existing liver disease, the use of potentially hepatotoxic drugs, systemic inflammatory response during infection, respiratory distress syndrome that could induce hypoxia, and multiple organ dysfunction were among them, but it seems to be multifactorial.
Several reports mentioned increased liver enzyme, especially in severe cases [14–16] and in a study done in the PICU of our centre most of the patients had elevated liver enzyme, especially those with a poor outcome [17].
In the present study, 75.4% of our patients had elevated AST and 44.5% had increased ALT, in line with the retrospective study by Hundt MA et al. on 1,827 adult patients with Covid-19 [18]. They reported elevated AST in 66.9%, ALT in 41.6%, decreased albumin in 56.7% and high total bilirubin in 4.3% of the patients. Also, an association between abnormal liver function tests and severe Covid-19, including ICU admission, mechanical ventilation and death, was reported [18].
Another study showed that 46% of admitted patients infected with Covid‐19 had elevated AST and 35% had elevated ALT levels on admission [19]. In a study by Luglio M et al., the patients with liver damage had higher levels of inflammatory markers, such as CRP [20], but we saw no correlation between high CRP and elevated liver enzymes.
Other studies indicated AST elevations could occur in SARS-CoV-2 infections, even in the absence of previous hepatic disease [21,22]. A study of 148 patients showed that 37.2% of these patients had abnormal liver function tests and of these, the patients with abnormal tests had higher CRP [23]. Yang et al. showed that 29% of their patients had liver dysfunction but there was no difference in the incidences of abnormal liver function between survivors and non-survivors [22].
In our study, of the patients admitted to the PICU, 83.6% had abnormal AST and 50.7% had high ALT. Guan et al. performed a study in 1,099 patients. Elevated levels of AST were observed in 18.2% of patients with non-severe disease and 39.4% of patients with severe disease. Elevated levels of ALT were reported in 19.8% of patients with non-severe disease and in 28.1% of patients with severe disease [24].
Zhou YH et al. showed that the occurrence of Covid-19 related abnormal liver enzymes is more common in men. This increase in abnormal liver enzymes is not present in children [13]. A study of patients with chronic liver disease concluded that they do not have an increased risk of severe disease course of SARS-CoV-2 infection with little or no liver dysfunction [25]. Guan WJ et al. showed increased creatine phosphokinase in 14% of their patients [24].
In our study, an increased level of LDH and CRP and decreased albumin were related to the severity of the disease and mortality, in concordance with Zeng- -Hong Wu et al., who in a meta-analysis showed a significant relation between liver dysfunction and mortality from Covid-19 [26]. Aziz M et al., in a systematic review and meta-analysis showed that the mean serum albumin upon admission in severe cases was lower than in the non-severe Covid-19 patients [27]. In another study, it was shown that hypoalbuminaemia is associated with more unsatisfactory outcomes [28]. The major limitation of this study was that all the patients who were admitted to our study were not tested for liver function and could not enroll in the aforementioned study.
In conclusion, abnormal liver function tests are commonly seen in children with Covid-19 infection and we can consider hypoalbuminaemia in addition to high LDH and CRP as poor prognostic factors.
ORCID authors
A. Saeed ORCID 0000-0001-9864-3087,
A. Naghshzan ORCID 0000-0001-7647-178X,
S. M. Dehghani ORCID 000-0001-5930-01100.
Submitted/Doručeno: 13. 1. 2022
Accepted/Přijato: 9. 2. 2022
Seyed Mohsen Dehghani, MD, MPH
Department of Pediatrics
Division of Pediatric intensive care
Shiraz University of Medical Sciences
Namazi Hospital, Namazi square
Postal code: 7193613311
Shiraz, Zand, Iran
dehghanism@gmail.com
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Xu L, Liu J, Lu M et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020; 40(5): 998–1004. doi: 10.1111/liv.14435.
2. Gupta A, Madhavan MV, Sehgal K et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26(7): 1017–1032. doi: 10.1038/ s41591-020-0968-3.
3. Duan Z, Chen Y, Zhang J et al. Clinical characteristics and mechanism of liver injury in patients with severe acute respiratory syndrome. Zhonghua Gan Zang Bing Za Zhi 2003; 11(8): 493–496.
4. Arabi YM, Al-Omari A, Mandourah Y et al. Critically Ill patients with the middle east respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med 2017; 45(10): 1683–1695. doi: 10.1097/CCM.0000000000002621.
5. Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382(18): 1708–1720. doi: 10.1056/NEJMoa2002032.
6. Ding Y, He L, Zhang Q et al. Organ distribution of severe acuterespiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 2004; 203(2): 622–630. doi: 10.1002/path.1560.
7. Li W, Moore MJ, Vasilieva N et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426(6965): 450–454. doi: 10.1038/nature02145.
8. Hoffmann M, Kleine-Weber H, Krüger N et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv 2020 [ahead of print]. doi: 10.1101/2020.01.31.929042.
9. Chai X, Hu L, Zhang Y et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020 [ahead of print]. doi: 10.1101/2020.02.03.931766.
10. Bjornsson ES. Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci 2016; 17(2): 224. doi: 10.3390/ijms17020224.
11. Saeed A, Shorafa E, Shahramian I et al. An 11-year-old boy infected with COVID-19 with presentation of acute liver failure. Hepat Mon 2020; 20(6): e104415. doi: 10.5812/hepatmon.104415.
12. Viswanathan V, Save S, Sawant V et al. Hepatitis: an emerging presentation in child with coronavirus disease 2019. Indian J Child Health 2021; 8(3): 139–141. doi: 10.32677/IJCH.2021.v08.i03.008.
13. Zhou YH, Zheng KI, Targher G et al. Abnormal liver enzymes in children and infants with COVID-19: a narrative review of case-series studies. Pediatr Obes 2020; 15(12): e12723. doi: 10.1111/ijpo.12723.
14. Henry BM, Benoit SW, de Oliveira MHS et al. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): a pooled analysis and review. Clin Biochem 2020; 81: 1–8. doi: 10.1016/j.clinbiochem.020.05.012.
15. Qi K, Zeng W, Ye M et al. Clinical, laboratory, and imaging features of pediatric COVID-19: a systematic review and meta-analysis. Medicine (Baltimore) 2021; 100(15): e25230. doi: 10.1097/MD.0000000000025230.
16. Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med 2020; 58(7): 1135–1138. doi: 10.1515/cclm-2020-0272.
17. Saeed A, Shorafa E, Sanaeidashti A et al. Clinical presentation of paediatric patients with COVID-19 admitted to a single paediatric intensive care unit (PICU) in Iran. BMJ Paediatr Open 2020; 4(1): e000715. doi: 10.1136/bmjpo- 2020-000715.
18. Hundt MA, Deng Y, Ciarleglio MM et al. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. Hospital Network. Hepatology 2020; 72(4): 1169–1176. doi: 10.1002/hep.31487.
19. Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res 2020; 25(1): 54. doi: 10.1186/s40001-020-00454-x.
20. Luglio M, Tannuri U, de Carvalho WB et al. COVID-19 and liver damage: narrative review and proposed clinical protocol for critically ill pediatric patients. Clinics (Sao Paulo) 2020; 75: e2250. doi: 10.6061/clinics/2020/e2250.
21. Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020; 5(5): 428–430. doi: 10.1016/S2468-1253(20)30057-1.
22. Yang X, Yu Y, Xu J et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8(5): 475–481. doi: 10.1016/S2213-2600(20)30079-5.
23. Fan Z, Chen L, Li J et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol 2020; 18(7): 1561–1566. doi: 10.1016/j.cgh.2020.04.002.
24. Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382(18): 1708–1720. doi: 10.1056/NEJMoa2002032.
25. Di Giorgio A, Hartleif S, Warner S et al. COVID-19 in children with liver disease. Front Pediatr 2021; 9: 616381. doi: 10.3389/fped.2021.616381.
26. Huang J, Cheng A, Kumar R et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol 2020; 92(10): 2152–2158. doi: 10.1002/jmv.26003.
27. Aziz M, Fatima R, Lee-Smith W et al. The association of low serum albumin level with severe COVID-19: a systematic review and meta-analysis. Crit Care 2020; 24(1): 255. doi: 10.1186/s13054-020-02995-3.
28. Bertolini A, van de Peppel IP, Bodewes FAJA et al. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology 2020; 72(5): 1864–1872. doi: 10.1002/hep.31480.