Hepatitis B in persons infected with the human immunodeficiency virus in Porto-Novo – prevalence and associated factors
Finagnon Armand Wanvoegbe1, Aboudou Raïmi Kpossou2, Cossi Angelo Attinsounon3, Djimon Marcel Zannou4, Kouessi Anthelme Agbodandé4, Donalds Ahogbehossou1, Adebayo Alassani3, Angèle Azon-Kouanou1, Albert Dovonou3, Jean Sehonou2, Fabien Houngbe4
+ Affiliation
Summary
Introduction: Approximately 5 to 25% of the 34 million people living with HIV (PLHIV) worldwide are co-infected with hepatitis B virus (HBV) or hepatitis C virus. The objective of this work was to determine the prevalence of HBsAg (Hepatitis B surface Antigen) in PLHIV at the University Hospital of Porto-Novo and to identify the factors associated with HIV-HBV co-infection. Methods: This was a descriptive and analytical cross-sectional study covering the period from 1/2/2016 to 1/5/2016. PLHIV individuals with or without antiretroviral therapy (ARV) who were older than 15 years and had given their consent were included in the study. HBsAg was investigated by the DiaSpot One-Step Test Strip Rapid Test. Results: In the study participated 301 PLHIV individuals. The average age was 42 ± 10.3 years old. The age groups 35–45 (39.2%) and 25–35 (23.9%) were the most represented. Women (73.1%) were predominant. The main common risk factors related to HIV infection were unprotected sex (98%), multiple sexual partners (57.5%), circumcision outside hospital (66.7% of men), scarification (78.1%) and transfusion (17.6%). The prevalence of HBsAg was 14.3%. Factors associated with HIV-HBV co-infection were a history of liver disease and alanine aminotransferase levels more than twice the normal level. Conclusion: The prevalence of HBsAg was high in our PLHIV group. Local authorities in Benin should instigate measures to make HbsAg testing systematic during initial assessments of patients in order to adapt ARV treatment to each case.
Keywords
Benin, HIV-HBV, co-infection, seroprevalenceIntroduction
Sub-Saharan Africa continues to bear the brunt of the global epidemic of HIV/AIDS. In 2013, United Nations Programme on HIV/AIDS (UNAIDS) estimated there were 24.7 (23.5–26.1) million people living with HIV (PLHIV). In that same year, 70% of new infections worldwide (1.5 million) were in this region and 210,000 new HIV infections were observed in children [1]. HIV mortality from opportunistic infections has declined significantly over the last two decades, due to the success of highly active antiretroviral therapy. In contrast, chronic liver disease is increasingly recognised as a leading cause of morbidity and mortality in HIV-infected patients co-infected with hepatitis B vi-rus (HBV) or hepatitis C virus (HCV) [2,3]. Infection with HBV and HCV is also a major public health problem in southern countries. One of the areas of higher prevalence of HBV is West Africa and the situation is comparable for HCV [4]. Infected at an early age, infection with HBV is at high risk of chronic disease with the possibility of progression to cirrhosis or primary liver cancer [5]. Globally, the seroprevalence of HIV-HBV co-infection is estimated at between 2 and 4 million people and that of HIV-HCV co-infection is estimated at between 4 and 5 million [6,7]. World Health Organization recommends testing for HBV and HCV before initiating antiretroviral therapy for PLHIV [8]. However, we find that HBV testing among PLHIV is not systematic in Benin, because it was not part of the package supported free of charge by the state. This study was therefore undertaken to supplement existing HBV seroprevalence data in adults living with HIV in Benin. Our objective was to determine the seroprevalence of HBV in PLHIV followed in the Department of Internal Medicine of the University Hospital Center Ouemé-Plateau (CHUD-O/P) and to identify the factors associated with this HIV-HBV co-infection.
Methods
It was a descriptive and analytical cross-sectional study covering the period from 1/2/2016 to 1/5/2016. Comprehensive recruitment of all people living with HIV followed as outpatients or hospitalised in the CHUD-O/P, at least 15 years old and given informed consent.
The diagnosis of hepatitis B in our study was based on the detection of HBsAg. For the detection of HBsAg, the DiaSpot One-Step Test Strip rapid test was used.
Data were collected through a standardised questionnaire divided into three parts:
- the first part concerned the data collected during the direct interview with the patient;
- the second focused on blood test results;
- the third was data collected from the patient‘s medical record.
Data were entered using the EPIINFO version 7 software after checking each record; they were analysed using the EPIINFO version 7 and STATA 11 software. The proportions were compared using the Chi2 test and the Prevalence Report. A threshold of significance of 5% was used. The word processor, tables and graphs were made using Microsoft Word 2013 and Microsoft Excel 2013 software.
Results
A total of 301 PLHIV met our inclusion criteria during the study period. They had all been looking for HBsAg and were our study sample.
Sociodemographic characteristics
The average age was 42 years ± 10.3 years, with extremes of 19 and 74 years. The most represented age groups were 35 to 45 years old (39.2%) and 25 to 35 years old (23.9%). This population was predominantly female (73.1%), with a sex ratio of 0.37. Regarding common risk factors, 98% of respondents had unprotected sex, 57.5% had multiple sexual partners, 66.7% of men were circumcised outside hospital, 0.5% of women were excised, 78.1% had scarifications, 17.6% have been transfused at least once.
Prevalence of HIV-HBV co-infection
Of the 301 PLHIV surveyed, 43 were co-infected with HBV, which gave a prevalence of 14.3%.
Analysis of factors associated with co-infection
The analysis was done to investigate the socio-demographic, clinical, immunological, virological and biochemical factors of the PLHIV surveyed related to HIV-HBV co-infection.
Sociodemographic factors associated with HIV-HBV co-infection
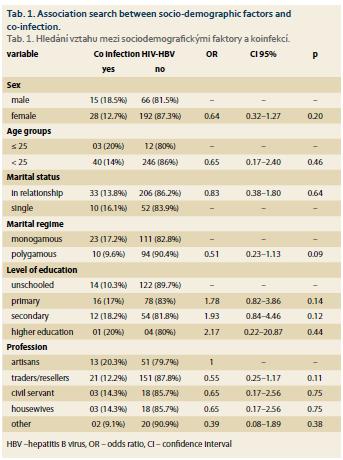
Tab. 1 presents the relationship between socio-demographic factors and HIV-HBV co-infection. None of these factors were significantly associated with HIV-HBV co-infection among our respondents.
History of HIV-HBV co-infection
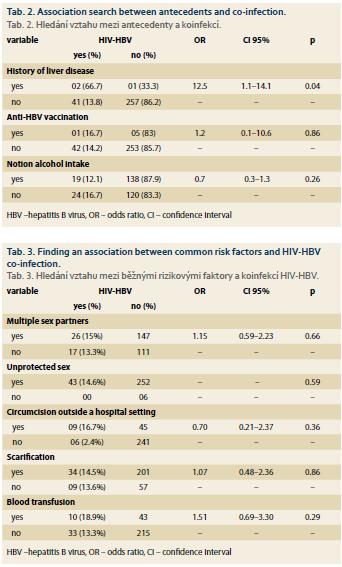
Tab. 2 shows the relationship between antecedents and HIV-HBV co-infection. Only the history of previous hepatic pathology was significantly associated (p = 0.04) with HIV-HBV co-infection. HIV-HBV co-infection was higher among respondents with a history of liver disease compared to those who did not.
Common Risk Factors associated with HIV-HBV co-infection
Tab. 3 presents the relationship between common risk factors and HIV-HBV co-infection.
None of these factors were significantly associated with HIV-HBV co-infection.
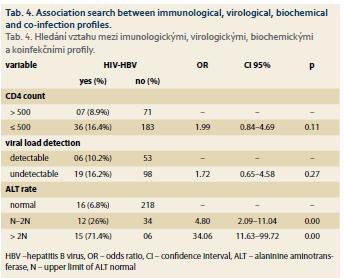
Virological and biochemical immunological profiles associated with HIV-HBV co-infection
Tab. 4 shows the relationship between immunological, virological, biochemical profiles and HIV-HBV co-infection. Alanine aminotransferase (ALT) was significantly associated with HIV-HBV co-infection. Respondents with ALT between N (N = upper limit of ALT normal) and 2N were more HIV-HBV co-infected than those with a normal rate. Respondents with ALT > 2N were more HIV-HBV co-infected than those with a normal rate.
Discussion
The prevalence of HBsAg in the population of our study is 14.3%. As screening for HBsAg may mask false negatives in occult hepatitis in PLHIV, this high prevalence is likely to be underestimated. The detection of viral load of HBV would be the only method that would make it possible to evaluate the true proportion of our study population carrying HBV. This high prevalence confirms the geographical distribution of HBV endemicity, according to which the prevalence of HBV is 8% or more in the Sub-Saharan region [9].
In Benin, the prevalence of HBsAg among PLHIV, found in a prior study in Cotonou, was comparable to ours, as was that found in Parakou. Indeed Sehonou et al. [10] in 2007 reported a prevalence of 11.21% in the antiretroviral patient population at National University Hospital Center in Cotonou. Dovonou et al. [11] in 2012 found a prevalence of 16.8% in the population of patients living with HIV and monitored at University Hospital Borgou-Alibori in Parakou. Our prevalence is also comparable to those found by Jobarteh et al. [12] (12.2%) in HIV-infected patients of the Genitourinary Clinic in The Gambia, Adewole et al. [13] (11.5%) among PLHIV at the Abuja National Hospital in Nigeria, Diope-Ndiaye et al. [14] (16.8%) in the cohort of the Senegalese ARV Access Initiative in Senegal, Nagu et al. [15] (17.3%) among PLHIV at the Muhimbili National Hospital in Tanzania. Although high, our result was still lower than those of Tounkara et al. [16] (25.3%) in HIV-infected blood donors in Mali and Lukhwareni et al. [9] (22.9%) in South Africa in the population of PLHIV in a tertiary hospital. Our result remains higher than those found in Europe, the United States and Asia. Indeed, Konopnicki et al. [17] reported in the Euro AIDS (Acquired Immune Deficiency Syndrome) cohort a prevalence of 8.7%. Hun Kim et al. [18] found a prevalence of 4.47% among PLHIV at St. Luke Roosevelt Hospital in New York. Yan et al. [19] reported a prevalence of 12.49% in China among PLHIV from infectious disease hospitals in seven provinces with high HIV prevalence. Koike et al. [20] found in Japan a prevalence of 6.3% among PLHIV across 372 HIV/AIDS network hospitals. This variation in the seroprevalence of HBsAg confirms the heterogeneity of the geographical distribution of HBV in the world.
None of the sociodemographic factors studied in our work were statistically associated with the HIV-HBV co-infection state. According to Dovonou et al. [11] the driving profession was significantly exposed to this comorbidity, the male subject, aged 15 to 25, living in a couple in a polygamous relationship was most affected by the HIV-HBV co-infection. In the study of Sehonou et al. [10] no socio-demographic factor was associated with co-infection. Diop-Ndiaye et al. [14] also found significant male predominance (23.7 vs. 11.5%; p <0.05).
Clinically, 66.7% of patients with a history of liver disease were found to be significantly HBV carriers. It should be noted that the risk of progression from HBV infection to chronicity was higher among PLHIV [21].
Only six (2%) of the 301 PLHIV surveyed in the study had received at least one dose of hepatitis B vaccine and one of these six were HIV-HBV co-infected. This low percentage of vaccination in the population would probably be due to the lack of knowledge of the disease or the lack of information and awareness in the general population about the existence of the vaccine.
Unprotected sex and multi-sexual partnership was a risky behaviour found in our general population increasing exposure to HIV-VHB co-infection. In our study, 14.6% and 15% respectively of subjects having unprotected sex and multiple sexual partners were HIV-HBV co-infected, but unprotected sex and multi-sexual partnership were not significantly associated with HIV-HBV co-infection (p > 0.05). Scarification was also one of the risk factors for transmission exposing these two viruses, but not significantly: 14.5% of scarification patients were co-infected (p > 0.05). Although risk factors for contamination in our study population – circumcision outside the hospital setting (16.7%) and blood transfusion (18.9%) – were not significantly associated with co-infection. Homosexuality has not been noted in our population. This could be explained in two ways: either it was absent from the population, or it existed but would not be revealed by the subjects concerned because of our socio-cultural realities. In fact, homosexuals are individuals rejected by our society. Dovonou et al. [11] found that unprotected sex, multiple sexual partnership and scarification were risk factors that significantly increased co-infection. For Sehonou et al. [10] the risk factors significantly increasing exposure to co-infection were blood transfusion and acupuncture. Adewole et al. [13] noted risk factors which increase exposure to co-infection: multi-sex partnership, scarifications and repeated transfusions. According to Shimelis et al. [22] risk factors for transmission were sexually transmitted infections (STIs) (54% of patients with a history of STIs were co-infected, p < 0.05), surgery (56.1% of patients with antecedent of surgery are co-infected, p < 0.05) and catheterisation (61.7% of patients with a history of catheterisation are co-infected, p < 0.05). Koike et al. [20] found the risk factors exposing injecting drug use (66.7%, p < 0.05), blood transfusion (40%, p < 0.05) and homosexuality (32.02%, p < 0.05). According to Konopnicki et al. [17] the risk factors for both viruses were homosexuality (49.8%), injecting drug use (25.7%) and heterosexuality (16.5%, p < 0.05).
The common risk factors for transmission of HIV and HBV according to these different studies therefore varied according to the population studied and the socio-cultural realities specific to each region. In our study population, socio-economic and cultural realities would promote multi-sexual partnership, low intellectual level would be the basis of unprotected sex and customs and traditional medicine associated with scarification and circumcision outside hospital.
Hepatic cytolysis was not common, but ALT was significantly associated with HIV-HBV co-infection. The risk of cytolysis with ALT greater than 2N was 34-fold higher in co-infected patients than in mono-infected patients. This would probably be the witness of a pro-gressive disease favoured by immunosuppression due to HIV in these subjects co-infected with HBV. Dovonou et al. [11] found that cytolysis was rare and the risk of cytolysis with ALT greater than 2N was 5-fold higher in co-infected patients than in mono-infected patients. Biochemically, they found that there was no significant difference between mono and co-infected patients. Sehonou et al. [10] found a result similar to that of Dovonou et al. biochemically; hepatic cytolysis was rare and was not statistically associated with HIV-HBV co-infection.
Conclusion
Our study of HBV co-infection among PLHIV followed in the Department of Internal Medicine CHUD-O/P shows that the prevalence of HBsAg is high (14.3%) in this population. However, this prevalence would be underestimated, as we did not combine hepatitis B core antigen antibody testing and HBV viral load detection to determine the true proportion of co-infected individuals in our population. Therefore, local authorities in Benin should make the search for HBsAg systematic in PLHIV for appropriate treatment.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Submitted/Doručeno: 3. 4. 2018
Accepted/ Přijato: 17. 9. 2018
Aboudou Raïmi KPOSSOU, MD
University Clinic of Hepatogastroenterology
National University Hospital Center
(CNHU-HKM) of Cotonou
kpossou.raimi@yahoo.fr
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Organisation des nations unies pour le sida (ONUSIDA). Raport Mondial. Rapport ONUSIDA sur l‘épidémie mondiale de sida 2013. [online]. Available from: http: //www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Report_2013_fr_1.pdf.
2. Bonacini M, Louie S, Bzowej N et al. Survival in patients with HIV infection and viral hepatitis B or C: a cohort study. AIDS 2004; 18 (15): 2039–2045.
3. Ioannou GN, Bryson CL, Weiss NS et al. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 2013; 57 (1): 249–257. doi: 10.1002/hep.25800.
4. Organisation mondiale de la Santé. Assemblée mondiale de la santé journal. Soixante-Troisième Assemblée mondiale de la Santé. Point 11.12, Hépatite virale. [online]. Available from: http: //apps.who.int/gb/ebwha/pdf_files/WHA63/J_No1_2010-fr.pdf.
5. Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis 2007; 7 (6): 402–409. doi: 10.1016/S1473-3099 (07) 70135-4.
6. Reinert P, Si-Ali A, Mongin C et al. Hepatitis special. Développement et santé 2012; 200: 1–80. [online]. Available from: https: // studylibfr.com/doc/6550814/consulter-la-ressource.
7. Lambotin M, Barth H, Moog C et al. Challenges for HCV vaccine development in HIV-HCV coinfection. Expert Rev Vaccines 2012; 11 (7): 791–804. doi: 10.1586/erv.12.52.
8. World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach. Geneva: WHO 2010; 1–63.
9. Lukhwareni A, Burnett RJ, Selabe SG et al. Increased detection of HBV DNA in HBsAg-positive and HBsAg-negative South African HIV/AIDS patients enrolling for highly active antiretroviraltherapy at a Tertiary Hospital. J Med Virol 2009; 81 (3): 406–412. doi: 10.1002/jmv.21 418.
10. Sehonou J, Fiogbe AA, Zannou DM et al. Co-infection VIH-VHB chez les patients sous traitement antiretroviral au Centre Hospitalier et Universitaire de Cotonou (République du Bénin). Rev CAMES-Série A 2010; 10: 85–89.
11. Dovonou CA, Amidou SA, Kpangon AA et al. Prévalence de l’hépatite B chez les personnesinfectées par le VIH à Parakou au Bénin. Pan Afr Med J 2015; 20: 125. doi: 10.11604/pamj.2015.20.125.6061.
12. Jobarteh M, Malfroy M, Peterson I et al. Seroprevalence of hepatitis B and C virus in HIV-1 and HIV-2 infected Gambians. Virol J 2010; 7: 230. doi: 10.1186/1743-422X-7-230.
13. Adewole OO, Anteyi E, Ajuwon Z et al. Hepatitis B and C virus co-infection in Nigeria patients with HIV infection. J Infect Dev Ctries 2009; 3 (5): 369–375.
14. Diop-Ndiaye H, Touré-Kane C, Etard JF et al. Hepatitis B, C seroprevalence and delta viruses in HIV-1 Senegales patients at HAART initiation (retrospective study). J Med Virol 2008; 80 (8): 1332–1336. doi: 10.1002/jmv.21 236.
15. Nagu TJ, Bakari M, Matee M. Hepatitis A, B and C viral co-infections among HIV-infected adults presenting for care and treatment at Muhimbili National Hospital in Dar es Salaam, Tanzania. BMC Public Health 2008; 8: 416. doi: 10.1186/1471-2458-8-416.
16. Tounkara A, Sarro YS. Seroprevalence of HIV/HBV coinfection in Malian blood donors. J Int Assoc Physicians AIDS Care (Chic) 2009; 8 (1): 47–51. doi: 10.1177/1545109708330 118.
17. Konopnicki D, Mocroft A, De Wit D et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005 March; 19 (6): 593–601.
18. Kim JH, Psevdos G, Suh J et al. Co-infection of hepatitis B and hepatitis C virus in human immunolodeficiency virus-infected patients in New York City, United States. World J Gastroenterol 2008; 14 (43): 6689–6693.
19. Yan YX, Gao YQ, Sun X et al. Prevalence of hepatitis C virus and hepatitis B virus infections in HIV-positive Chinese patients. Epidemiol Infect 2011; 139 (3): 354–360. doi: 10.1017/S0950268810001597.
20. Koike K, Tsutsumi T, Kikuchi Y et al. Prevalence of hepatitis B virus infection in Japanese patients with HIV. Hepatol Res 2008; 38 (3): 310–314. doi: 10.1111/j.1872-034X.2007.00 263.x.
21. EASL. EASL clinical practice guidelines. Management of chronic hepatitis B. Gastroenterol Clin Biol 2009; 33 (6–7): 539–554. doi: 10.1016/j.gcb.2009.03.002.
22. Shimelis T, Torben W, Medhin G et al. Hepatitis B virus infection among people attending the voluntary counselling and testing centre and anti-retroviral therapy clinic of St Paul’s General Specialised Hospital, Addis Ababa, Ethiopia. Sex Transm Infect 2008; 84 (1): 37–41. doi: 10.1136/sti.2007.027326.