Evaluation of the association between red cell distribution width (anisocytosis) and degree of liver fibrosis in children with chronic liver diseases
Seyed Mohsen Dehghani1, Masoud Tahani2, Mahammad Reza Bordbar1, Mahsa Borjzadeh Gashtaseb1, Iraj Shahramian1
+ Affiliation
Summary
Background: Red cell distribution width (RDW) demonstrates the heterogeneity of red cell volume and is a component of the complete blood count. Recent studies, however, have reported that RDW is associated with increased mortality in many clinical conditions and found that high RDW is associated with an increase in all-cause mortality. Some studies have also reported the association between RDW values and the severity of liver diseases. It has been claimed that elevated RDW values positively correlate with MELD scores in different disease statuses of hepatitis B virus infection. In addition, RDW increased with the worsening of Child-Pugh grade in hepatic cirrhosis. Methods: This study investigated the clinical utility of RDW values for indicating the presence of liver fibrosis in children with chronic liver diseases. We have conducted a retrospective study on 413 patients. We collected demographic, clinical, and laboratory data and pathologic reports of the liver fibrosis stage from the medical record and analyzed them with SPSS. Result: In our study, there was no significant association between the values of RDW and different stages of fibrosis, but the association between the values of RDW and worsening of Child-Pugh score, APRI, RPR, FIB-4, and PELD score was significant. Conclusion: We cannot find any correlation between RDW and the stage of liver fibrosis.
Keywords
cirrhosis, liver, fibrosis, RDW
Introduction
Regardless of cause, chronic hepatitis is defined as an ongoing condition that has not improved for at least six months, although the diagnosis can be made earlier in certain cases [1]. The most frequent causes of chronic hepatitis are autoimmune hepatitis (AIH), hepatitis B, and hepatitis C [2–5]. A major cause of abnormal liver function tests is chronic hepatitis, which also serves as the background for the progression of cirrhosis and hepatocellular cancer. Liver biopsy remains the gold standard method for assessing liver histology, although it is costly, invasive, and has a risk of complications with morbidity between 0.3 and 0.6% and mortality of 0.05%. Moreover, liver biopsy requires hospitalization of at least 6-18 hours. Additionally, sample errors and inter- and intra-observer differences might lead to under-staging of cirrhosis, particularly macronodular cirrhosis [6–10].
In children, a wide range of causes of hepatocellular injury may result in cirrhosis [11]. These include causes of cholestasis, where there is an accumulation of hydrophobic bile acids toxic to hepatocytes (e. g., biliary secretory disorders or obstruction), infections, toxins, and metabolic, vascular, and nutritional disorders. Anisocytosis is said to be present when the red cells vary significantly in size [12]. One prominent cause of cirrhosis in children is biliary atresia, a condition characterized by the absence or obstruction of the bile ducts, resulting in impaired bile flow. This condition stands as the most prevalent cause of liver cirrhosis among children, as the progressive cholestasis and inflammation associated with biliary atresia can eventually lead to liver fibrosis and cirrhosis. Similarly, Alagille syndrome, a genetic disorder affecting the bile ducts, heart, skeleton, and other organs, can contribute to chronic cholestasis, ultimately leading to liver fibrosis and cirrhosis in affected children.
Another genetic disorder, alpha-1 antitrypsin deficiency, disrupts the production and function of the protein alpha-1 antitrypsin. This abnormal accumulation of the protein within liver cells can result in liver damage, inflammation, and eventual cirrhosis. Furthermore, Wilson disease, an inherited condition characterized by impaired copper metabolism, leads to the accumulation of copper in various organs, including the liver. Left untreated, this buildup of copper can cause liver cell damage and progress to cirrhosis.
Infections also play a significant role in pediatric chronic hepatitis and subsequent cirrhosis. Hepatitis B virus (HBV) is a common culprit, often transmitted from infected mothers to their babies during childbirth or through close contact with infected individuals. Chronic HBV infection can trigger liver inflammation and fibrosis over time. Similarly, hepatitis C virus (HCV) infection, primarily acquired through exposure to infected blood products or vertical transmission from infected mothers, can result in chronic hepatitis and potentially progress to cirrhosis. Additionally, autoimmune hepatitis (AIH), an immune-mediated disorder, can provoke chronic hepatitis in children by causing the immune system to mistakenly attack liver cells, leading to inflammation and liver damage [13–15].
When considering the pathophysiological explanations for the findings about RDW, it becomes evident that there are intriguing factors to explore. While the precise mechanisms remain partially elusive, there are several potential explanations for the correlation between RDW and liver fibrosis. One plausible theory is that liver fibrosis induces alterations in the bone marrow microenvironment, thereby influencing the production and maturation of red blood cells. Impaired liver function can result in reduced erythropoietin production, leading to ineffective erythropoiesis and subsequent variations in red blood cell size and volume.
Furthermore, the presence of chronic inflammation and oxidative stress, which are commonly observed in liver diseases, can also impact the morphology and functionality of red blood cells. The release of inflammatory cytokines and markers of oxidative stress during liver fibrosis may exert effects on red blood cell membrane integrity, ultimately contributing to increased variability in cell size [16,17].
Greater variability in shapes of red blood cells and accompanying increased number of abnormally shaped cells is called poikilocytosis. The electronic cell counter provides an independent assessment of variability in red cell size. It measures the range of red cell volumes and reports the results as “red cell distribution width” (RDW). This value is calculated from the MCV; thus, cell width is not being measured, but cell volume is. The term is derived from the curve displaying the frequency of cells at each volume, also called the distribution. The red cell volume distribution curve’s width determines the RDW. The RDW is calculated as follows: RDW = (standard deviation of MCV ÷ mean MCV) × 100 [13–15]. In Xu WS and coworkers’ study, they divided 446 hepatitis B virus-infected patients who underwent liver biopsy into two groups: absent or mild and moderate–severe according to the severity of liver fibrosis and inflammation. They found that RDW values increase with progressive liver fibrosis and inflammation. Laboratory parameters, including TBIL, ALT, AST, MPV, and RDW (list of abbreviations at the end of the article), increased with progressive fibrosis stages, whereas Alb and PLT counts were inversely related to stages. Also, they found that APRI had an excellent predictive value for significant liver necrosis and inflammation but exhibited a poor diagnostic value for predicting fibrosis [18,19]. Chen B et al divided 458 patients with chronic hepatitis B who had undergone liver biopsy into two cohorts: an estimation group (N = 310) and a validation group (N = 148). They assessed liver histology according to the Metavir scoring scheme. They showed that RPR could predict significant fibrosis and cirrhosis in patients with chronic hepatitis B, potentially reducing the number of unnecessary liver biopsies [20]. According to Huang R and coworkers‘ study RDW was positively correlated with worsening Child-Pugh scores and MELD scores [21]. To the best of our knowledge, the correlation between RDW and the extent of liver fibrosis has not been extensively investigated, and the impact of liver fibrosis on blood parameters remains unknown. Hence, the objective of this study is to assess the relationship between red cell distribution width and the degree of liver fibrosis in pediatric patients with chronic liver diseases.
Nevertheless, it is essential to underscore the significance of further research in order to fully comprehend the underlying mechanisms that establish the association between RDW and liver fibrosis. Conducting additional studies that focus on exploring the specific pathways and interactions involved will undoubtedly contribute to a more comprehensive understanding of this intricate relationship.
Methods and material
We have done a retrospective study. All children under 18 with chronic liver disease who underwent liver biopsy in the Pediatric Gastroenterology and Hepatology Ward, Namazi Teaching Hospital, affiliated with Shiraz University of Medical Sciences from March 2014 to March 2017 were enrolled in this retrospective study. All children with chronic liver disease but without a liver biopsy are excluded from the study. Other exclusion criteria are infectious diseases on admission, chronic renal diseases, collagen vascular diseases, malignancies, hematological diseases, hemoglobinopathies, and blood transfusions. Chronic liver disease is diagnosed by clinical, biochemical, radiographic, and histopathologic findings of liver biopsies. Demographic, clinical, and laboratory data were collected from the patient’s medical records. A nearest complete blood count (CBC) to the time of liver biopsy containing RDW, hemoglobin, mean corpuscle volume (MCV), white blood cell, platelets, and mean platelet volume (MPV) was extracted from their medical files. The Beckman Coulter automated analyzer calculates RDW as RDW = SD of MCV / mean of MCV × 100. The SD represented the standard deviation of the volume of erythrocytes or RBCs in the blood smear. The liver function tests include total bilirubin, albumin, INR, alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum sodium, and creatinine extracted from their files. Child-Pugh and PELD/MELD scores are calculated for all cirrhotic patients. Also, APRI, AAR, RPR, and also FIB-4 are calculated for all patients as below:
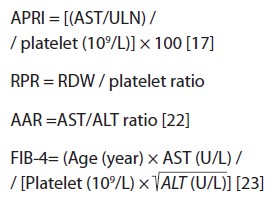
ULN of AST is considered 31 for females and 41 for males based on our laboratory cut-offs.
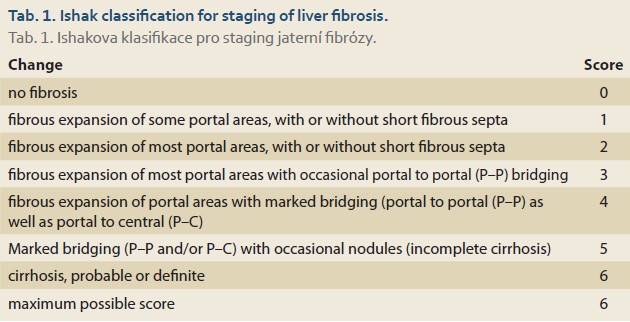
Ishak classification is used for staging liver fibrosis. Stage 0 (no fibrosis); 1 (expansion of some portal areas with or without septa); 2 (expansion of most portal areas with or without septa); 3 (expansion of most portal areas with an occasional portal to portal bridging); 4 (expansion of portal areas with marked bridging; a portal to portal and/or portal to central); 5 (marked bridging with occasional nodules; incomplete cirrhosis), and 6 (cirrhosis, probable or definitive) (Tab. 1) [24].
Fibrotic stages 0–2, 3 and 4, and 5 and 6 are defined as mild, moderate, and severe fibrosis, respectively. Statistical analyses are performed using the SPSS 19 software. Continuous variables are displayed as mean ± standard deviation or median (25th, 75th percentile). Categorical variables are shown as numbers and percentages. Normally distributed and parametric variables are compared between groups using the one-way ANOVA test where appropriate. One-way ANOVA assesses the correlation between RDW and liver histopathological features (inflammation, degree of fibrosis). Pearson correlation is used to assess the association between RDW and PELD/MELD score. P <0.05 is considered statistically significant.
Results
In total, 413 patients were enrolled in our study between March 2014 and March 2017. Two hundred nineteen were male (53%), and 194 were female (47%). 48.18% of the patients were 1 year old, and 51.82% were 1 to 18 years old.
361 of cases were under 12 years old (87.4%), and 52 were 12 years old (12.6%)
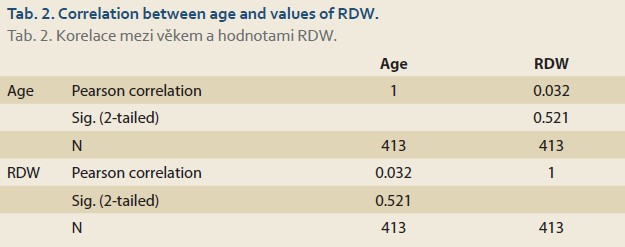
In our study, there was no correlation between age (patients all aged less than 18 years old) and values of RDW using Pearson correlation (P value = 0.521) (Tab. 2).
The underlying diseases of the patients and the number of patients afflicted with them are listed below (Tab. 3).
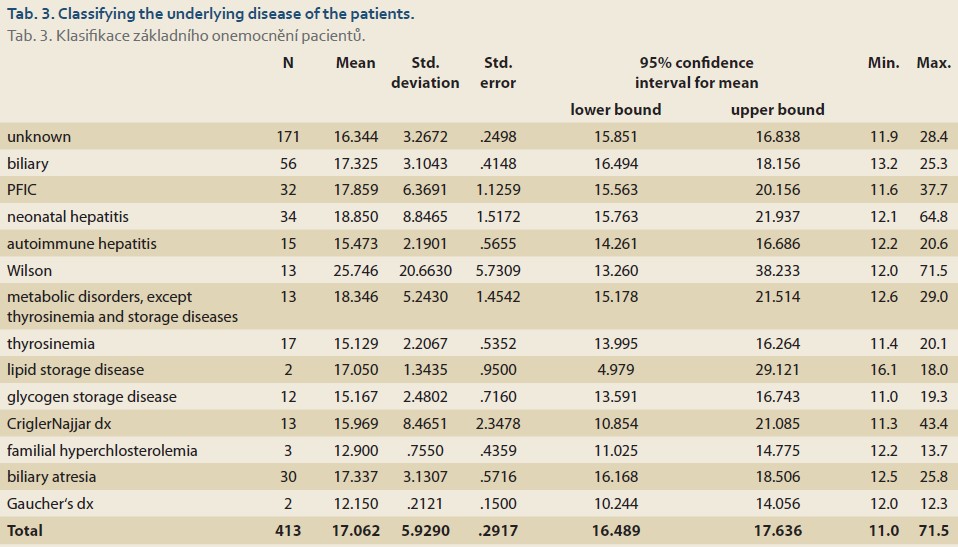
Using the one-way ANOVA test, there was a significant association between the patient‘s underlying diseases and values of RDW (P value <0.001).
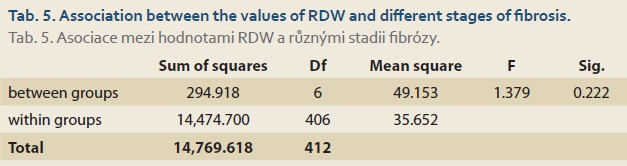
There was also a significant association between the underlying diseases of the patients and different stages of fibrosis (from 0 to 6) using the Chi-square test (P value ≤0.001). 92.3% of patients with Wilson disease and 88.2% of patients with thyrosinemia were at stage 6 of fibrosis (Tab. 4). There was no significant association between the amounts of the RDW and different stages of fibrosis (P value = 0.278) (Tab. 5).
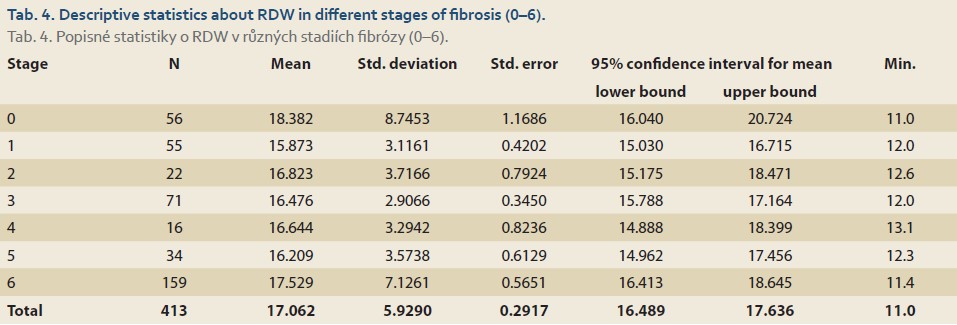
Fibrotic stages 0–2, 3 and 4, and 5 and 6 are defined as mild, moderate, and severe fibrosis, respectively. RDW decreased linearly from the mild to the moderate stage and increased linearly from the moderate to the severe stage; thus, overall, there was no association between RDW and mild, moderate, and severe stages of fibrosis.
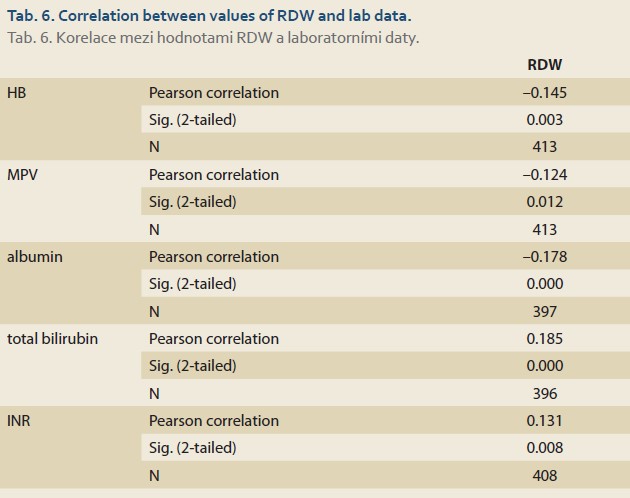
Based on the Ishak classification, there was a significant association between MCV, platelet, albumin, INR, and creatinine values and different fibrosis stages [25]. RDW was positively correlated with total bilirubin and INR and was negatively correlated with hemoglobin, MPV, and albumin using Pearson correlation for analysis (Tab. 6).
There was a significant association between RPR and different stages of fibrosis with one-way ANOVA test. (P value <0.001) RPR decreased linearly from the mild to moderate fibrosis stage and increased linearly from the moderate to severe. There was no significant association between AAR and different stages of fibrosis with one-way ANOVA test. (P value = 0.382) There was a significant association between APRI and different stages of fibrosis with one-way ANOVA test. (P value = 0.001) APRI decreased linearly from the mild to moderate fibrosis stage and increased linearly from the moderate to severe.
There was a significant association between the value of FIB-4 and different fibrosis stages with a one-way ANOVA test (P value <0.001) (Tab. 7).
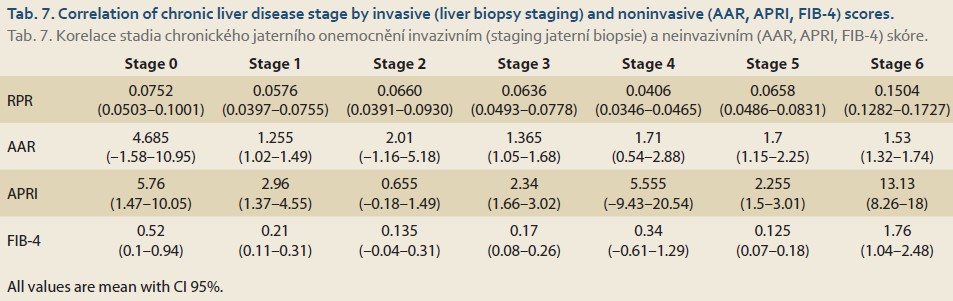
FIB-4 decreased linearly from mild to moderate stage of fibrosis and increased linearly from moderate to severe fibrosis. There was a significant association between RDW and PELD score (P value <0.001), but there was no significant association between RDW and MELD score based on Pearson correlation (P value = 0.124).
There was a significant correlation between WBC, hemoglobin, MCV, the value of RDW, platelet, MPV, albumin, total bilirubin, INR, AST, ALT, Cr, and Child- -Pugh scores (A, B, and C) as listed below: (Worsening of Child-Pugh score positively correlates with MCV, RDW, total bilirubin, INR, AST, and ALT and negatively correlates with hemoglobin and albumin.) WBC, platelet, and MPV increased from A to B score and decreased from B to C.Cr decreased from A to B score and increased B to C score.
Discussion
At present, the liver biopsy is a gold standard for diagnosis of liver fibrosis [26,27], but it has some potential complications such as hemorrhage, hematoma, creating an arteriovenous fistula, pneumothorax, or bile peritonitis [21] that have limited the usage of this method. For this reason, many studies have been conducted to find noninvasive markers to diagnose liver fibrosis.
Although Hu Z‘s study showed that RDW increases in patients with liver disease [28], in our study, there was no association between the value of RDW and different stages of fibrosis in children with chronic liver diseases, consistent with the Hu Z et al. study [28], we found that RDW increases with worsening Child-Pugh scores. There was also a significant association between other laboratory findings: (WBC, hemoglobin, MCV, platelet, MPV, albumin, total bilirubin, INR, AST, ALT, creatinine) with Child--Pugh scores. Worsening of Child-Pugh score positively correlates with MCV, RD with W, Total bilirubin, INR, AST, and ALT, and negatively correlates with hemoglobin and albumin. WBC, platelet, and MPV increased from A to B score and decreased from B to C. Cr decreased from A to B score and increased from B to C score. Our study significantly associated RPR, APRI, FIB-4, and fibrosis stages. However, no association between AAR and different stages of fibrosis is somehow consistent with Abdollahi M and coworkers‘ study that concluded that FIB-4 and APRI were superior to AAR at distinguishing severe fibrosis from mild-to-moderate fibrosis [29].
Despite Huang R and coworkers‘ study that concluded that there is an association between MELD score and grading of fibrosis, we did not find any significant association between them but an association between RDW and PELD score (P value <0.001) [21].
Conclusion
No correlation between RDW and liver fibrosis stage was discovered in our investigation, despite several studies showing one. The variability of the study participants‘ medical conditions may be one of the causes of the variations in the research findings. Consider the patients‘ varying ages in different research. It is advised that more research be carried out to more accurately identify whether RDW can be utilized as a marker.
Abbreviations
MCV mean corpuscular volume
TBIL total bilirubin
ALT alanine aminotransferease
AST aspartate aminotransferase
MPV mean platelet volume
Alb albumin
PLT platelets
APRI AST to platelet ratio index
RPR rapid plasma reagin
ULN upper limit of normal
ORCID authors
S. M. Dehghani ORCID 0000-0001-5930-0110,
M. Bordbar ORCID 0000-0002-8071-6425,
M. B. Gashtaseb ORCID 0009-0006-5272-6788,
I. Shahramian ORCID 0000-0002-4134-1405,
M. Tahani ORCID 0000-0002-0213-9087.
Submitted/Doručeno: 8. 1. 2023
Accepted/Přijato: 25. 5. 2023
Masoud Tahani, MD, PhD.
MSC of Clinical Biochemistry
Pediatric Gastroenterology, and Hepatology Research Center
Zabol University of Medical Sciences
Shahid Rajaei Street
9861615881 Zabol
Iran
masood.tahani@gmail.com
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Hudacko R, Theise N. Liver biopsies in chronic viral hepatitis: beyond grading and staging. Archives of pathology & laboratory medicine. Arch Pathol Lab Med 2011; 135(10): 1320–1328. doi: 10.5858/arpa.2011-0021-RA.
2. Ghany MG, Strader DB, Thomas DL et al. Diag- nosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49(4): 1335–1374. doi: 10.1002/hep.22759.
3. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45(2): 507–539. doi: 10.1002/hep. 21513.
4. Czaja AJ. Performance parameters of the conventional serological markers for autoimmune hepatitis. Digestive diseases and sciences. Dig Dis Sci 2011; 56(2): 545–554. doi: 10.1007/s10620-010-1501-1.
5. Abdollahi MR, Somi MH, Faraji E. Role of international criteria in the diagnosis of autoimmune hepatitis. World J Gastroenterol 2013; 19(23): 3629–3633. doi: 10.3748/wjg.v19.i23.3629.
6. Berasain C, Betes M, Panizo A et al. Pathological and virological findings in patients with persistent hypertransaminasaemia of unknown aetiology. Gut 2000; 47(3): 429–435. doi: 10.1136/gut.47.3.429.
7. Hano H, Takasaki S. Three-dimensional observations on the alterations of lobular architecture in chronic hepatitis with special reference to its angioarchitecture for a better understanding of the formal pathogenesis of liver cirrhosis. Virchows Arch 2003; 443(5): 655–663. doi: 10.1007/s00428-003-0843-x.
8. Degos F, Perez P, Roche B et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010; 53(6): 1013–1021. doi: 10.1016/j.jhep.2010.05.035.
9. Poynard T, Imbert-Bismut F, Munteanu M et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol 2004; 3(1): 8. doi: 10.1186/1476-5926-3-8.
10. Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 2000; 32(3): 477–481. doi: 10.1053/jhep.2000.16602.
11. Kleinman RE. Walker’s pediatric gastrointestinal disease. USA: PMPH 2018.
12. Heimbach JK, Kulik LM, Finn RS et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67(1): 358–380. doi: 10.1002/hep.29086.
13. Yeung CY, Lee HC, Chan WT et al. Vertical transmission of hepatitis C virus: Current knowledge and perspectives. World J Hepatol 2014; 6(9): 643–651. doi: 10.4254/wjh.v6.i9.643.
14. Tian L, Ye Z, Kafka K et al. Biliary atresia relevant human induced pluripotent stem cells recapitulate key disease features in a dish. J Pediatr Gastroenterol Nutr 2019; 68(1): 56–63. doi: 10.1097/MPG.0000000000002187.
15. Perlmutter DH. Liver injury in alpha 1-antitrypsin deficiency: an aggregated protein induces mitochondrial injury. J Clin Invest 2002; 110(11): 1579–1583. doi: 10.1172/JCI16787.
16. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352(10): 1011–1023. doi: 10.1056/NEJMra041809.
17. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood 2019; 133(1): 40–50. doi: 10.1182/blood-2018-06-856500.
18. Kliegman RM, Behrman RE, Jenson HB et al. Nelson textbook of pediatrics e-book. Elsevier Health Sciences 2016.
19. Wyllie R, Hyams JS. Pediatric Gastrointestinal and Liver Disease E-Book: Expert Consult-Online and Print. Elsevier Health Sciences 2016.
20. Kasper DL, Fauci AS, Hauser SL et al. Harrison’s principles of internal medicine. McGraw Hill Education 2018: 379–387.
21. Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med 2014; 52(9): e197–199. doi: 10.1515/cclm-2014-0353.
22. Xu W-S, Qiu X-M, Ou Q-s et al. Red blood cell distribution width levels correlate with liver fibrosis and inflammation: a noninvasive serum marker panel to predict the severity of fibrosis and inflammation in patients with hepatitis B. Medicine (Baltimore) 2015; 94(10): e612. doi: 10.1097/MD.0000000000000612.
23. Chen B, Ye B, Zhang J et al. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One 2013; 8(7): e68780. doi: 10.1371/journal.pone.0068780.
24. Huang R, Yang C, Wu K et al. Red cell distribution width as a potential index to assess the severity of hepatitis B virus‐related liver diseases. Hepatol Res 2014; 44(14): E464–E470. doi: 10.1111/hepr.12342.
25. Ishak K, Baptista A, Bianchi et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6): 696–699. doi: 10.1016/0168-8278(95)80226-6.
26. Berger D, Desai V, Janardhan S. Con: liver biopsy remains the gold standard to evaluate fibrosis in patients with nonalcoholic fatty liver disease. Clin Liver Dis (Hoboken) 2019; 13(4): 114–116. doi: 10.1002/cld.740.
27. Mahjoubin-Tehran M, De Vincentis A, Mikhailidis DP et al. Non-alcoholic fatty liver disease and steatohepatitis: State of the art on effective therapeutics based on the gold standard method for diagnosis. Mol Metab 2021; 50: 101049. doi: 10.1016/j.molmet.2020.101049.
28. Hu Z, Sun Y, Wang Q et al. Red blood cell distribution width is a potential prognostic index for liver disease. Clin Chem Lab Med 2013; 51(7): 1403–1408. doi: 10.1515/cclm-2012-0704.
29. Abdollahi M, Pouri A, Ghojazadeh M et al. Noninvasive serum fibrosis markers: A study in chronic hepatitis. BioImpacts 2015; 5(1): 17–23. doi: 10.15171/bi.2015.05.