Endoscopic drainage of pancreatic fluid collections – 6 years’ experience at a tertiary referral gastroenterological center in a period 2006–2012
Martin Kliment Orcid.org 1, Ondřej Urban Orcid.org 2,3, Petr Fojtík Orcid.org 4, Martin Straka Orcid.org 5, Martin Loveček Orcid.org 6, Jaroslav Krátký Orcid.org 7, Pavol Holéczy Orcid.org 8,9
+ Affiliation
Summary
Introduction: Walled-off pancreatic fluid collections, that are categorized as pancreatic pseudocysts and walled-off pancreatic necrosis (WOPN), arise as complications of acute or chronic pancreatitis. Endoscopic transmural drainage (ETD) is the current method of choice for the treatment of symptomatic walled-off pancreatic fluid collections. Methods: A retrospective analysis of prospectively collected data from patients who underwent an ETD at a tertiary referral gastroenterological center during a 6-year interval was performed. The technical, short-and long-term clinical success of ETD of walled-off pancreatic fluid collections was evaluated. The effectiveness of ETD performed with two types of endoscopes, with a duodenoscope (CTD – conventional transmural drainage) and with an echoendoscope (EUD – endoscopic ultrasound-guided drainage), was also compared. The choice of technique was at the discretion of the endoscopist performing the procedure. Results: In a cohort of 54 patients with a symptomatic pseudocyst or WOPN, the overall technical success rate of ETD was 94.4%, and this did not differ between EUD (100%) and CTD (88.6%) when the appropriate technique was selected (p = 0.104). Complications occurred in 31.5% of the patients, with no difference found between CTD and EUD (38.5 vs. 25%; p = 0.382). Clinical success persisted 3 months after stent insertion in 92.5% of patients with initial technical success of ETD and 6 months after stent extraction in 88.6% of the patients with clinical success 3 months after stent insertion. No difference was observed between EUD and CTD (3 months after stent insertion: 90.5 vs. 94.8%; p = 1.0; 6 months after stent extraction: 100 vs. 77.8%; p = 0.104). Of 51 patients with initial technical success, the therapeutic effect 6 months after stent extraction was 62.7%. Conclusion: In a cohort of 54 patients, the technical success, short-term and long-term clinical success of ETD of walled-off pancreatic fluid collections was 94.4, 92.5 and 88.6%, respectively. Endoscopic ultrasound plays an important role in this therapy when gastric varices or a non-bulging fluid collection is involved. Furthermore, no difference was observed between EUD and CTD in terms of technical and clinical success when the appropriate technique was selected.
Keywords
acute pancreatitis, endoscopic drainage, endosonography, walled-off pancreatic necrosis, pancreatic pseudocystIntroduction
Acute pancreatitis (AP) is the leading gastroenterological cause of patients’ hospital admission [1]. Some local complications of AP, such as infected walled-off pancreatic necrosis (WOPN) or pseudocyst, significantly increase the hospital length of stay, morbidity and mortality of the patients [1]. Four types of fluid collections associated with AP are presently distinguished [2]:
1. acute peripancreatic fluid collection (APFC),
2. acute necrotic collection (ANC),
3. pseudocyst,
4. WOPN.
Classification criteria for fluid collections associated with AP include both the time period after the onset of AP and the presence of necrosis inside the collection. APFC and ANC develop during the first 4 weeks after the onset of AP and are not walled-off. Pseudocyst and WOPNs develop in the period of > 4 weeks after the onset of AP and contain a defined wall [2]. They are jointly referred to as walled-off pancreatic fluid collections in the text below.
Treatment is only indicated for symptomatic walled-off fluid collections, namely pseudocysts and WOPNs.
In addition to AP, pseudocysts occur in 20–40% of patients with chronic pancreatitis (CP) [3].
Until recently, the treatment of walled-off pancreatic fluid collections had been the domain of surgeons. With a developement of therapeutic endoscopy, it has been moved into the hands of interventional endoscopists. Before the introduction of therapeutic endoscopic ultrasound (EUS), endoscopic transmural drainage (ETD) of pancreatic pseudocysts was performed using a duodenoscope. This technique, however, can only be used if the gastro-intestinal (GI) wall is bulging under pressure from the pseudocyst, and in the absence of gastric varices. Such limitations do not apply to ETD performed with a therapeutic echoendoscope. In 1992, Grimm et al. [4] performed the first EUS-guided transmural drainage of a pseudocyst in the tail of the pancreas using a linear echoendoscope in a patient with CP.
The primary objective of our study was to evaluate the technical and clinical success of ETDs of walled-off pancreatic fluid collections, including the incidence of complications. The secondary objective was to compare those parameters between the sub-group of patients with a drainage performed with the use of a duodenoscope (CTD – conventional transmural drainage) and the subgroup of patients with a drainage performed with the use of an echoendoscope (EUD – endoscopic ultrasound-guided drainage).
Patients and methods
We have performed a retrospective analysis of a prospectively collected data from patients who underwent an ETD of walled-off pancreatic and/or peripancreatic fluid collection (in the following text referred to as walled-off pancreatic fluid collection) in the Digestive Disease Center in Vítkovice Hospital in the period from August 2006 to July 2012. The majority of patients had a pancreatic pseudocyst and a minority had WOPN. Both groups of patients were analysed together, since WOPN had not yet been defined at the time of data collections. In the years 2006–2007, therapeutic EUS was not yet available at the author’s workplace and during this period drain-ages were only performed as CTDs. Since 2008, therapeutic EUS became a part of endoscopic procedures performed at the author’s workplace and echoendoscope (EUD) began to be used in ETD, too. In the following years, both techniques (CTD and EUD) were used to perform ETDs and the choice of technique was at the discretion of the endoscopist performing the ETD. Initially, that choice could have been affected by the endoscopist’s experience with each technique, as well as by the fact that EUS belonged not to a standard equipment in an endoscopic suite with a fluoroscopy (equipped with a C-arm X-ray machine). However, most of the ETDs were performed under the fluoroscopy just in this room. EUD was primarily preferred in the absence of a visible bulge in the GI wall, or in the presence of gastric varices. In case of a technical failure of the first technique, the other technique was employed at the endoscopist’s discretion. CTD was performed with a duodenoscope (Olympus JGF 160, Olympus Europe, Hamburg, Germany) and EUD was performed with a linear echoendoscope (Olympus GF UCT 140 AL, Olympus Europe, Hamburg, Germany). An abdominal computed tomography (CT) scan was performed in all patients before ETD to rule out a pseudoaneurysm or an interposition of a blood vessel between the GI wall and the wall of the fluid collection. Once EUS has been fully established at the author’s workplace, all patients underwent an endoscopic ultrasound examination shortly before CTD to assess any presence of gastric and perigastric varices or an interposed vessel, as well as the distance between the fluid collection and GI walls. For an ETD to be performed safely, the required distance between the collection and GI walls was ≤ 1cm. At a later phase, after WOPN had been defined, the EUS examination also served to distinguish between a pseudocyst and WOPN, with ETD followed by endoscopic transmural necrectomy (ETN) in case of the latter. In some patients, an appropriate location for the drainage was marked in the stomach just before CTD, using echoendoscope and biopsy forceps. The need for such marking was established at the discretion of the endoscopist performing the ETD. ETD was performed under antibiotic prophylaxis after a single i.v. dose of amoxicilin-clavulanate or fluorochinolone just before the procedure, and under analgosedation with intravenously administered midazolam and fentanyl.
ETD procedure for pancreatic pseudocyst
At the beginning of the ETD procedure, an access into the pseudocyst cavity was achieved using a 10 Fr cystostome (CST-10, Cook Medical Europe, Limerick, Ireland). The created stoma was then dilated with a balloon of 8–10 mm in diameter (Boston CRE™ Wire-guided Esophageal, Boston Scientific, USA) and usually three double-pigtail plastic (DP) stents (also referred to as drain or drains in the following text) 5 cm long and 10 Fr in diameter (Advanix Double Pigtail, Boston Scientific, USA) were inserted transmurally into the pseudocyst cavity. The number of stents was established at the discretion of the endoscopist performing the ETD. The stents remained in place for a period of 3 months and in case of pseudocyst regression confirmed by follow-up CT scans or magnetic resonance imaging scans, alternatively magnetic resonance cholangiopancreatography scans, they were extracted using an endoscope and a polypectomy snare, once the disconnected pancreatic tail syndrome had been ruled out.
At the beginning of the study, there was neither a definition, nor a recommendation for an endoscopic management of WOPN. The Atlanta classification of AP was applied and WOPN management was identical to the management of pancreatic pseudocysts. As soon as the definition of WOPN and its adequate endoscopic therapy had been established, these have also been introduced and implemented at the author’s workplace.
ETD procedure for WOPN
In case of WOPN, characterised by the presence of a solid necrosis in the fluid collection cavity, the first step involved ETD, just as in the case of a pancreatic pseudocyst. However, during the ETD a 250cm long 7 Fr nasocystic drain (ENBD-7, Cook Medical Europe, Limerick, Ireland) was placed into the necrotic cavity, in addition to the three DP stents, to carry out a necrotic cavity lavage both by permanent application of saline (1,000 mL over 24 hours) as well as by bolus application of 200 mL of saline every 4–6 hours. ETN followed several (usually 3) days after the primary drainage. A therapeutic double-channel video gastroscope (Olympus GIF-2TH180, Olympus Europe, Hamburg, Germany) was used to perform the ETN. Two of the three primary DP stents were removed with a polypectomy snare (SD-5U-1, Olympus Europe, Hamburg, Germany). The stoma was then dilated with a balloon (Boston CRE™ Wire-guided Esophageal, Boston Scientific, USA) to 18–20 mm in diameter and endoscopic necrectomy was performed with a Dormia basket (MSB-3X6-6, Cook Medical Europe, Limerick, Ireland), a polypectomy snare or a retrieval mesh (Roth Net Platinum Retriever, US Endoscopy, Mentor, OH, USA). Once the necroses had been completely removed, the remaining procedures were identical to those following an endoscopic drainage of pseudocysts, including follow-up imaging examinations and DP stents extraction 3 months after the primary drainage.
After the removal of DP stents, the majority of patients were followed-up at the outpatient clinic of the Digestive Disease Center at the Vitkovice Hospital, while the minority were followed-up by the gastroenterologists outside. A follow-up examination at 6 months after stents extraction included a medical history, a physical examination and an abdominal ultrasound. For patients monitored by other practices, information concerning the patient’s condition and the results of abdominal ultrasound examination were obtained by contacting the referring gastroenterologist.
Technically successful ETD was defined as the insertion of ≥ 1 DP stent into the fluid collection cavity. Short-term clinical success was defined as a resolution of the clinical symptoms with the regression of the fluid collection to a maximum diameter of < 3 cm at 3 months after DP stents insertion, i.e. just before their extraction. Long-term clinical success was defined in the same way but in a time frame of 6 months after the extraction of stents. ETD complications were defined as any ETD-related change in the pa-tient’s health condition requiring a treatment or a prolonged hospital stay.
We have evaluated the technical success, short-term and long-term clinical success of ETDs of all fluid collections, i.e. pseudocysts and WOPN together, performed with both techniques. Then we compared the above listed effectiveness parameters for ETDs performed with the duodenoscope and the echoendoscope (CTD vs. EUD).
Statistical analysis
Microsoft Excel 2003 software (Microsoft Corporation, Redmond WA, USA) was used for data storage and basic analysis. Statistical analysis was performed using SPSS v. 14.0 (SPSS Inc., Chicago, IL, USA). Differences in categorical variables of technical success, complications, short-term and long-term clinical success between the EUD and CTD groups were analysed using Fisher’s exact test, with p < 0.05 value being considered statistically significant.
Results
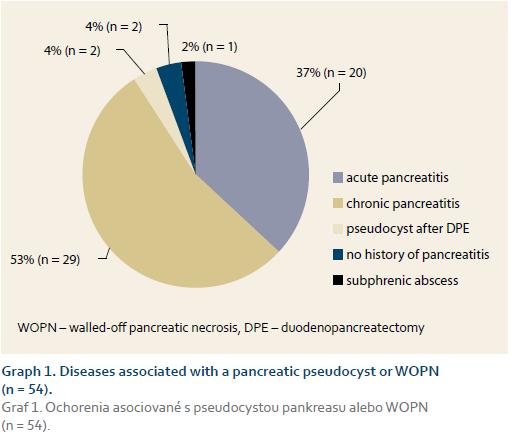
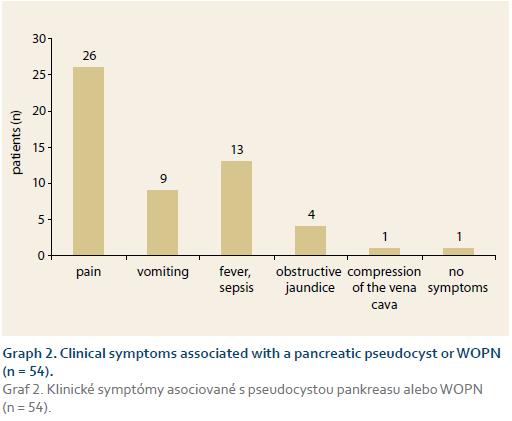
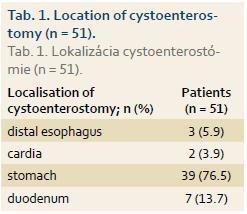
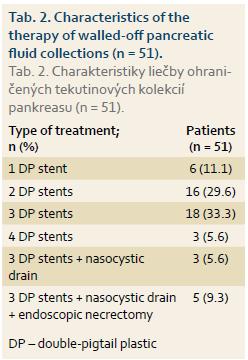
Of the total of 54 patients (mean age ± SD: 51 ± 10.6 years, range 35–72 years, 68.5% male) 28 (52%) underwent EUD and 26 (48%) CTD. ETD was initially technically unsuccessful in 5 patients, in 2 of whom it was then successfully performed at a second session (EUD: 1 patient, CTD: 1 patient). ETD was therefore technically success-ful in 51 of 54 (94.4%) patients. Of the whole study group, 30 (55.6%) patients underwent an endoscopic retrograde pancreatography in addition to ETD, while transpapillary drainage of the pancreatic duct was performed in 6 (11%) patients. Diseases associated with pancreatic pseudocyst or WOPN are shown in Graph 1. Clinical symptoms associated with pseudocyst or WOPN are shown in Graph 2. Cystenterostomy location is shown in Tab. 1. The mean diameter of the fluid collection (± SD) was 105 mm (± 14.1 mm) (range 50–230 mm). In the whole study group, a pseudocyst was presented in 48 (88.9%) patients (7 of whom had an infected pseudocyst, referred to as abscess according to the original Atlanta classification) and WOPN was present in 6 (11.1%) patients. Characteristics of the pseudocyst and WOPN management are shown in Tab. 2.
Technical success of ETD
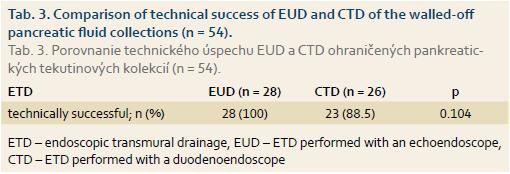
ETD of the pancreatic fluic collections was technically successful in 51 of 54 (94.4%) patients, with 2 of the successful ETDs only performed at a second session. There was no significant difference in the technical success between EUDs and CTDs, as shown in Tab. 3.
Complications
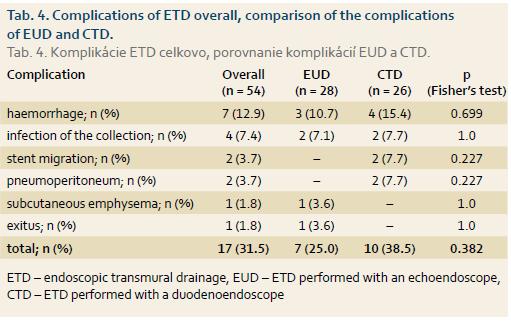
Of the total of 54 patients, a complication occurred in 17 (35%). The most common complication was bleeding (n = 7; 12.9%). One patient died suddenly on day 3 after an uneventful ETD and the cause of death was confirmed by section to be unrelated to ETD. Complications of ETD in the whole group, along with the incidence of complications following EUD and CTD, are shown in Tab. 4.
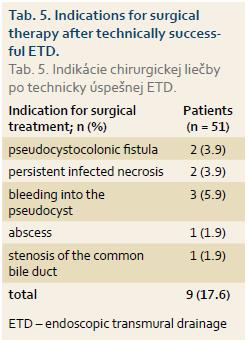
Of the 51 patients who have had a successful ETD of pancreatic fluid collection, 9 (17.6%) patients underwent surgery shortly after ETD for indications shown in Tab. 5.
Short-term clinical success 3 months after stents insertion
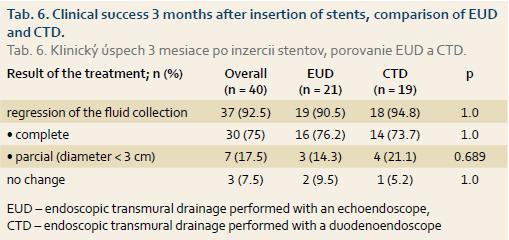
The analysis was performed in a group of 40 patients (9 patients who received surgery, as described above, were excluded from the group of patients with initially technically successfull drainage and 2 patients were lost to follow up). All patients had an abdominal CT scan at 3 months after the ETD. Regression of the fluid collection with a resolution of clinical symptoms was observed in 37 of the 40 (92.5%) patients who were followed up. Of the 3 (7.5%) patients with an unchanged pseudocyst 1 received surgery, 1 received endoscopic therapy and 1 was observed only.
In the group of 51 patients with the initially technically successful drainage, the clinical success at 3 months after ETD amounted to 72%. The clinical success at 3 months after ETD was not different between the two drainage techniques and is shown in Tab. 6.
Clinical success 6 months after stents extraction
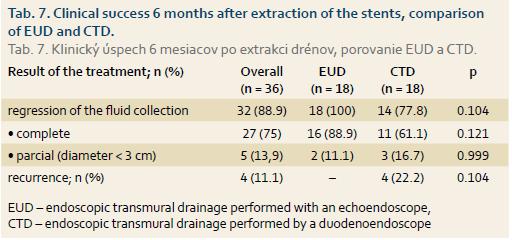
The analysis was performed in a group of 36 patients (1 of the 37 patients with the therapeutic success at 3 months after stents insertion was lost to follow-up). Four of 36 (11.1%) patients had a recurrence of pseudocyst at 6 months after stents extraction. Their relapse was managed as follows: ETD: 2 (5.6%) patients; surgery: 1 (2.8%) patient; percutaneous drainage: 1 (2.8%) patient. The therapeutic effect of ETD was thus maintained for 6 months after drain extraction in 32 of the 36 (88.9%) patients who had been successfully treated and achieved regression of the pseudocyst at 3 months after the drainage. With-in the group of 51 patients who had a technically successful drainage initially, the therapeutic success at 6 months after stents extraction represented 62.7% (32 of 51).
At 6 months after stents extraction, EUD was clinically more successful than CTD (Tab. 7), however, the difference was not statistically significant.
Discussion
Treatment options for walled-off pancreatic fluid collections include surgical drainage and resection (open or laparoscopic), endoscopic internal drainage and external percutaneous drainage. Due to its rather high morbidity (25%) and mortality (5%), open surgical drainage (cystogastro-stomy, cystoenterostomy) is nowadays hardly ever used [5]. Laparoscopic cystogastrostomy with an access from the lesser curvature is technically easier and carries a lower risk of bleeding [6]. Percutaneous CT-guided drainage is as effective as the surgical therapy [7]. The main complications of CT-guided drainage include the infection of the drain channel, occurring in up to 50% of patients [8], and pancreaticocutaneous fistula, which develops in up to 45% of patients, in particular in cases of a communication between pancreatic pseudocyst and a pancreatic duct, or in the presence of a stricture or a complete disruption of the main pancreatic duct [9]. Recently, ETD is an effective and a minimally invasive option for the treatment of pancreatic fluid collections. In the literature, its technicall success is reported to be > 90%, with the resolution of collections in 70–80% of patients [10,11]. Recurrence occurs in 10–15% of patients with a pseudocyst and in up to 30% of patients with WOPN [11]. ETD success rate depends on the type of fluid collection [11], reaching 74% for pseudocysts after AP, 92% for pseudocysts as complications of CP, and 72% for WOPN. Lower effectiveness of ETD in the management of WOPN is the result of a higher incidence of infectious complications, as well as difficulties with the removal of solid necrosis.
Short-term clinical success of ETD, defined as a reduction in size of the pancreatic fluid collection to < 3cm with the resolution of clinical symptoms, was maintained in our group at 3 months after stents placement in 92.5% (37 of 40) patients, while no difference was observed between CTD and EUD. Assessing the regression of the fluid collection at that time point is important for taking the decision about whether to extract or to leave the drains in place. In a randomised study by Park et al. [12], pseudocyst resolution was maintained at 1 month in 97% of patients after EUD and in 91% of patients after CTD.
Long-term clinical success of ETD of pancreatic fluid collections at 6 months after stents extraction was maintained in our group in 62.7% of patients who had initially a technically successful drainage. In the whole group of 54 patients, this represents a long-term clinical success of ETD of 59.3% (32 of 54), which is lower than in the reported literature [11].
In our study group, the lower long-term effectiveness of the therapy may have been caused by a relatively high proportion (11.1%) of WOPN. The managenemt of WOPN is different from the management of pseudocysts. At the early phase of this study, there was neither a definition of WOPN nor a recommendation for its management, including an endoscopic therapy. At that time, the Atlanta classification of AP was valid and the management of WOPN was at the author’s workplace identical to the management of pancreatic pseudocysts, which in the majority of cases led to the developement of a secondary infection.
Our study results may also have been affected by a gradually growing experience of the two endoscopists performing ETD (MK, OU) as the number of procedures increased. The loss of three patients with initially successful ETD during a follow-up period may also have had a negative impact on the overall clinical success of the therapy. Given that the endoscopic therapy was performed in a tertiary referral gastroenterological centre, it might be assumed that in case of a clinical failure those patients would have again been referred to the author’s workplace for further treatment. There-fore, assuming that the clinical effect of the therapy was maintained in those three patients at 6 months after stents extraction, the long-term clinical success would have increased to 68.6% (35 of 51) in the group of patients with a technically successful ETD and to 64.8% (35 of 54) in the whole study group.
For pancreatic pseudocysts, EUD is reported in the literature to be technically more successful than CTD. In 1 of the 2 randomised studies [12], technical success rate of EUD was 94% while the success rate of CTD was 72% (p = 0.039). Another randomised study [13] also confirmed a significantly higher technical success rate of EUD (100%) vs. CTD (33%; p < 0.001) in the management of pancreatic pseudocysts. In our study, the technical success rate was higher for EUD (100%) compared to CTD (88.6%), however, the difference was not statistically significant (p = 0.104). This was due to selection bias of ETD technique, depending on the presence of buldigng, gastric varices and availability of the endoscopic ultrasound. Moreover, the low number of patients and a possible statistical error type II might also have influenced these results. In two published randomised studies, CTD was unsuccessful in the absence of a buldge in the gastric wall produced by the pseudocyst. However, when both techniques were compared only in patients presenting with a buldging fluid collection, there was no difference in the technical success between EUD (100%) and CTD (100%) [12].
In our group, the overall complication rate was 31.5%. The risk of complications reported in the literature ranges from 11 to 37% [11–14], with a variability resulting from a lack of standardised follow-up and from different patient populations in the studies. Complications include intra-procedural and delayed bleeding, perforation, secondary infection and stent migration. The highest risk of complications is associated with the presence of a necrosis. Complications in our group, including bleeding and pneumoperitoneum, occurred more frequently after CTD (38.5%) than after EUD (25%), however, the difference was not statistically significant (p = 0.382). In the study by Park et al. [12], complications occurred in 7% of patients in the EUD group and in 10% of patients in the CTD group (p = 0.67). In another randomised study [13], there was also no difference in the rate of complications after EUD (0%) and CTD (13.3%; p = 0.48). Both complications after CTD were represented by bleeding, with one bleeding leading to a fatal outcome [13]. The reason for non-significance of the difference in the incidence of complications between conventional and EUS-guided ETD in our study, as well as in the published papers, is that the EUS-guided technique is selected in the presence of varices or in the absence of a bulge in the gastric wall. With a non-selective, random choice of technique in all patients, complications would have clearly occured more frequently after using the “blind” conventional technique (ETD).
In our group, a total of 11.1% of patients with a therapeutic success at 3 months after the insertion of DP stents experienced a recurrence of walled-off pancreatic fluid collection at 6 months after stents extraction, which occurred in 22.2% of patients in CTD group and none in EUD group (p = 0.104). In the literature, the recurrence rate of pseudocysts is reported to be 10–15% and of WOPN up to 30% [11]. In their study, Varadarajulu et al. [13] did not observe any diference in the therapeutic success between EUD and CTD. Similarly, Park et al. [12] reported, that the resolution of pancreatic pseduocysts was maintained at ≥ 6 months after the treatment in 97% of patients in the EUD group and in 91% in the CTD group (p = 0.565). The discrepancy between published studies and the result of our study in terms of long-term therapeutic success of EUD and CTD may have been caused by a higher proportion of patients with WOPN in the CTD group and a higher incidence of structural abnormalities of the pancreatic duct, predisposing to pseudocyst relapse if not corrected, in the CTD group.
In our cohort of patients, only DP stents were used in the ETD, because the special self-expanding metal stents with adapted ends (known as LAMS – lumen-apposing fully covered self-expanding metal stents) designed for draining pancreatic fluid collections (NAGI™ and SPAXUS™ stents by Tae Woong Medical, South Korea; AXIOS™ stent by Boston Scientific, USA) were not yet available at that time. Their main advantage is their simple and quick deployment, frequent spontaneous evacuation of necrosis through the lumen of the stent into the stomach/duodenum, and the possibility to perform endoscopic necrectomy directly through the lumen of the implanted stent. Their drawbacks include a high price and a risk of dislocation and bleeding after blood vessel arrosion inside WOPN. The number of studies comparing endoscopic therapy techniques for WOPN is still limited and none of them is a randomised comparative study.
In a recently published study [15] of 313 patients, there was retrospectively compared the effectiveness of endoscopic drainage of WOPN using DP stents (2 stents 10 Fr in diameter, n = 106), fully covered self-expanding biliary stents (FCSEMS, stent size 10 × 40 mm in 85 patients, 10 × 60 mm in 36 patients) and LAMS (AXIOS™ stent, n = 86). No differences in technical success were observed among the groups of patients. The incidence of early complications (≤ 7 days after stent placement) was significantly lower in the FCSEMS group than in the groups with drainage performed using DP stents and LAMS (1.6 vs. 7.5 vs. 9.3%; p < 0.01). Complete resolution of WOPN at 6 months after therapy was lower in the group with drainage performed using DP stents than in the FCSEMS and LAMS groups (81 vs. 95 vs. 90%; p = 0.01). The mean number of endoscopic procedures re-quired to achieve a complete WOPN resolution was significantly lower in the LAMS group compared to FCSEMS and DP stents groups (2.2 vs. 3 vs. 3.6; p = 0.04) [15]. This study has its limitations due to its retrospective design. Nevertheless, it provides important information concerning the effectiveness of endoscopic therapy for WOPN, with the highest effectiveness achieved, as expected, with LAMS. Moreover, it also highlights the potential risks and complications of this therapy, which are not negligible, given that the LAMS group showed the highest incidence of early complications, including bleeding (n = 6), superinfection (n = 1), perforation following incorrect AXIOS™ stent placement (n = 3) and abdominal pain (n = 1). Randomised studies, including a cost-benefit analysis, are required for a clear assessment of the benefits of therapy with the use of these special stents.
Conclusion
In a group of 54 patients, the technical success of ETD of walled-off pancreatic fluid collections was 94.4%. A complication occurred in 31.5% of patients of the whole study group, with bleeding occurring most frequently. Clinical effect of the therapy with resolution of fluid collection was maintained at 3 months after stents insertion in 92.5% of patients who had undergone a technically successful endoscopic drainage, and at 6 months after stents extraction in 88.6% of patients who showed clinical effect at 3 months after stents insertion. A reccurence of the fluid collection occurred in 11.1% of patients. With a selection of the technique, where EUD was predominantly used in patients with the absence of bulging and/or with the presence of gastric varices, no significant difference was observed between EUD and CTD in terms of technical success, the incidence of complications, or the clinical success. In view of the developments in therapeutic endoscopic ultrasound, EUD has now become a standard in the management of walled-off pancreatic fluid collections. New, specially adapted SEMS, known as LAMS, are most probably more effective than DP stents or FCSEMS in the endoscopic therapy of WOPN, although possibly at the cost of an increased risk of mainly bleeding complications. Randomised studies are required to assess their definitive role in the endoscopic management of walled-off pancreatic fluid collections.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Submitted/Doručeno: 25. 4. 2017
Accepted/Přijato: 11. 5. 2017
Martin Kliment, MD, PhD
Gastroenterologie und Hepatologie
Klinik für Innere Medizin
Vivantes Klinikum Spandau
Neue Bergstraße 6
135 85 Berlin
Germany
martin_kliment@hotmail.com
Literature
1. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastronterology 2013; 144 (6): 1252–1261. doi: 10.1053/j.gastro.2013.01.068.
2. Banks PA, Bollen TL, Dervenis C et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62 (1): 102–111. doi: 10.1136/gutjnl-2012-302779.
3. Usatoff V, Brancatisano R, Williamson RC. Operative treatment of pseudocysts in patients with chronic pancreatitis. Br J Surg 2000; 87 (11): 1494–1499.
4. Grimm H, Binmoller KF, Soehendra N. Endosonography guided drainage of a pancreatic pseudocyst. Gastrointest Endosc 1992; 38 (2): 170–171.
5. Bergman S, Melvin WS. Operative and nonoperative management of pancreatic pseudocysts. Surg Clin North Am 2007; 87 (6): 1447–1460.
6. Dávila-Cervantes A, Gómez F, Chan C et al. Laparoscopic drainage of pancreatic pseudocysts. Surg Endosc 2004; 18 (10): 1420–1426.
7. Szentes MJ, Traverso LW, Kozarek RA et al. Invasive treatment of pancreatic fluid collections with surgical and nonsurgical methods. Am J Surg 1991; 161 (5): 600–605.
8. Adams DB, Anderson MC. Percutaneous catheter drainage compared with internal drainage in the management of pancreatic pseudocyst. Ann Surg 1992; 215 (6): 571–576.
9. Fotoohi M, D’Agostino HB, Wollman B et al. Persistent percutaneous fistula after percutaneous drainage of pancreatic fluid collections: role of cause and severity of pancreatitis. Radiology 1999; 213: 573–578.
10. Baron TH. Endoscopic drainage of pancreatic pseudocysts, abscesses and organized (walled-off) necrosis. In: Baron TH, Kozarek R, Carr-Locke DL. ERCP. Philadelphia: Elsevier 2008: 475–493.
11. Baron TH, Harewood GC, Morgan DE et al. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc 2002; 56 (1): 7–17.
12. Park DH, Lee SS, Moon SH et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy 2009; 41 (10): 842–848. doi: 10.1055/s-0029-1215133.
13. Varadarajulu S, Christein J, Tamhane A et al. Prospective randomzed trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts. Gastrointest Endosc 2008; 68 (6): 1102–1111. doi: 10.1016/j.gie.2008.04.028.
14. Kahaleh M, Shami VM, Conaway MR et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy 2006; 38: 355–359.
15. Siddiqui AA, Kowalski TE, Loren DE et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: clinical outcomes and success. Gastrointest Endosc 2017; 85 (4): 758–765. doi: 10.1016/j.gie.2016.08.014.