Effect of ketamine, an NMDA-receptor antagonist, on gastric myoelectric activity in experimental pigs
Jan Bureš1,2,3, Jaroslav Květina Orcid.org 4, Věra Radochová5, Štěpán Suchánek6, Stanislav Rejchrt Orcid.org 4, Martin Vališ7, Veronika Knoblochová8, Jana Žďárová Karasová Orcid.org 9, Ondřej Soukup9, Darina Kohoutová1,10
+ Affiliation
Summary
Introduction: Preclinical studies in experimental pigs are carried out mostly under general anaesthesia. Ketamine is commonly used for induction of anaesthesia. However, there are concerns that ketamine, an NMDA-receptor antagonist, may influence gastric motor function. The aim of this study was to investigate porcine gastric myoelectric activity by means of electrogastrography (EGG). Methods: Seventeen female animals (mean weight 36.2±3.8 kg) were enrolled. Drugs used for induction of anaesthesia were: Group A (n=5): medetomidine 0.1 mg/kg i. m.; butorphanol 0.3 mg/kg i. m.; midazolam 0.3 mg/kg i. m.; Group B (n=6): azaperon 2.2 mg/kg i. m.; Group C (n=6): ketamine 20 mg/kg i. m.; azaperon 2.2 mg/kg i. m., followed in all groups by i.v. 1% propofol (repeated one-mL boluses, 10–12 mL in total). EGG recording started 15 min. after the induction administration and lasted 30 min. Results were evaluated as the dominant frequency of gastric slow waves (DF) and EGG power (areas of amplitudes). Results: In total, 510 one-minute EGG intervals were assessed. DFs were (mean ± standard deviation): 1.4±0.4 (Group A), 1.3±0.3 (Group B) and 0.2±0.1 cycles per min. (Group C). The differences between group C and groups A and B were statistically significant (p<0.001). Median power (IQR) was 0.13 (0.02–0.44; Group A), 0.13 (0.03–0.54; Group B) and 0.30 V2 (0.07–1.44; Group C). The difference between groups A and C was of borderline significance (p=0.066; type 2 error beta 0.295). Conclusions: Ketamine, administered even in a single intramuscular dose, affected myoelectric function of the porcine stomach. Therefore, it should be avoided in gastrointestinal motility studies in experimental pigs.
Keywords
ketamine, NMDA (N-methyl-D-aspartate) -receptor antagonist, electrogastrography, myoelectrical activity, experimental pig
Introduction
Gastric motor function is the most complex and most fragile function of the entire gastrointestinal tract [1]. Currently, there is no single gold standard for the diagnostics of gastric dysmotility disorders. It is possible to use gastric emptying scintigraphy, 13C-acetate or 13C-octanoic acid breath tests, electrogastrography, magnetic resonance imaging, antroduodenal manometry, ancillary testing (including barostat, SPECT, and satiety testing), wireless motility capsules, and last but not least EndoFLIP (Endoscopic Functional Lumen Imaging Probe: high-resolution impedance planimetry system) [2–23].
Electrogastrography (EGG) enables non-invasive recording of myoelectric activity of the stomach [24,25]. It is feasible both in humans [26–28] and in the experimental setting, including rats, rabbits, cats, dogs, and pigs [29–39]. Compared to humans, EGG in experimental pigs is the most reliable as the normal range of basic parameters is identical [40]. Previously, we have already presented an experimental porcine EGG in this Journal [41].
Nearly all preclinical porcine experiments must be carried out under general anaesthesia [42–45], and ketamine is commonly used as an induction of general anaesthesia in veterinary medicine [46–49].
In clinical practice, ketamine has been used since the 1970‘s for its anaesthetic, analgesic, antidepressant, and anti-inflammatory effect [50,51]. However, keta- mine also exerts several side effects in humans, such as dissociative and psychotomimetic effects, memory and cognitive impairment, as well as neurotoxicity, and is associated with a risk of abuse [50]. Ketamine is a phenylcyclohexylamine derivative (Fig. 1) [50].
Its mechanism of action is mainly explained by non-competitive antagonism of the N-methyl-D-aspartic acid (NMDA) receptor. Ketamine also interacts with opioid receptors and the monoamine, cholinergic, purinergic and adrenoreceptor systems, as well as having local anaesthetic effects [52].
However, ketamine, as an NMDA-receptor antagonist, might influence gastric myoelectric activity. The aim of this study was to compare the impact of ketamine and ketamine-free induction of general anaesthesia on EGG in experimental pigs.
Methods
Animals
In total, seventeen experimental adult female pigs (Sus scrofa f. domestica, hybrids of Czech White and Landrace breeds; 3-month-old; mean weight 36.2±3.8 kg; median 34.5 kg) were enrolled into the study. The animals were purchased from a certified breeder (Štěpánek, Dolní Ředice; SHR MUHO 2050/2008/41). The pigs were housed in an accredited vivarium (Faculty of Military Health Sciences, Hradec Králové). All animals were fed with a standard assorted A1 food (Ryhos, Nový Rychnov) in equal amounts twice a day, and had free access to drinking water.
Design of the study
All experiments were carried out in the morning on overnight fasting animals. Drugs used as an induction of anaesthesia were: Group A (n=5): medetomidine 0.1 mg/kg i. m.; butorphanol 0.3 mg/kg i. m.; midazolam 0.3 mg/kg i. m.; Group B (n=6): azaperon 2.2 mg/kg i. m.; Group C (n=6): ketamine 20 mg/kg i. m.; azaperon 2.2 mg/kg i. m.; followed in all groups by i.v. 1% propofol (repeated one-mL-boluses, 10-12 mL in total). EGG recording started 15 min. after the administration of induction and lasted 30 min. Heart rate monitoring and pulse oximetry were used to secure the experiments.
Electrogastrography
Our original method of porcine surface EGG was already published [53]. We used six active self-adhesive electrodes placed on the upper part of the abdomen, with the 7th basal electrode placed to the left of the middle of the sternum. A special abdominal belt enabled identification of artefacts caused by breathing and body movements. EGG recording was accomplished by means of the EGG Stand (MMS, Enschede, the Netherlands). Recordings were evaluated by MMS software (version 8.19). Running spectral analysis was used for standard evaluation of EGG. Results were conveyed as dominant frequency of gastric slow waves (DF; cycles per min.) and power analysis (areas of amplitudes; V2).
Statistics
Data was statistically treated by means of descriptive statistics, Fisher test, unpaired t-test, and Mann-Whitney rank sum test using SigmaStat software (Version 3.1, Jandel Corp, Erkrath, Germany). Type 2 error beta was calculated when appropriate.
Ethics
The Project was approved by the Institutional Review Board of the Animal Care Committee of the University of Defence (Protocol Number MO 171673/2019-684800), Faculty of Military Health Sciences, Hradec Králové. The study was conducted in accordance with the policy for experimental and clinical studies [45]. Animals were held and treated in conformity with the European Convention for the Protection of Vertebrate Animals [54].
'
Results
In total, 510 one-minute EGG intervals were assessed twice (DF and power). A total of 6 outliers (1.2% of all recordings; from various time intervals of different animals in all three groups) were excluded from the final evaluation of the EGG (two in DF and four in power analysis). An outlier is defined as a value outside the interval
[Q1 – 1.5 IQR, Q3 + 1.5 IQR], where Q1 is lower quartile, Q3 is upper quartile and IQR (= Q3 – Q1) is interquartile range. Major results are summarized in Fig. 2–11.
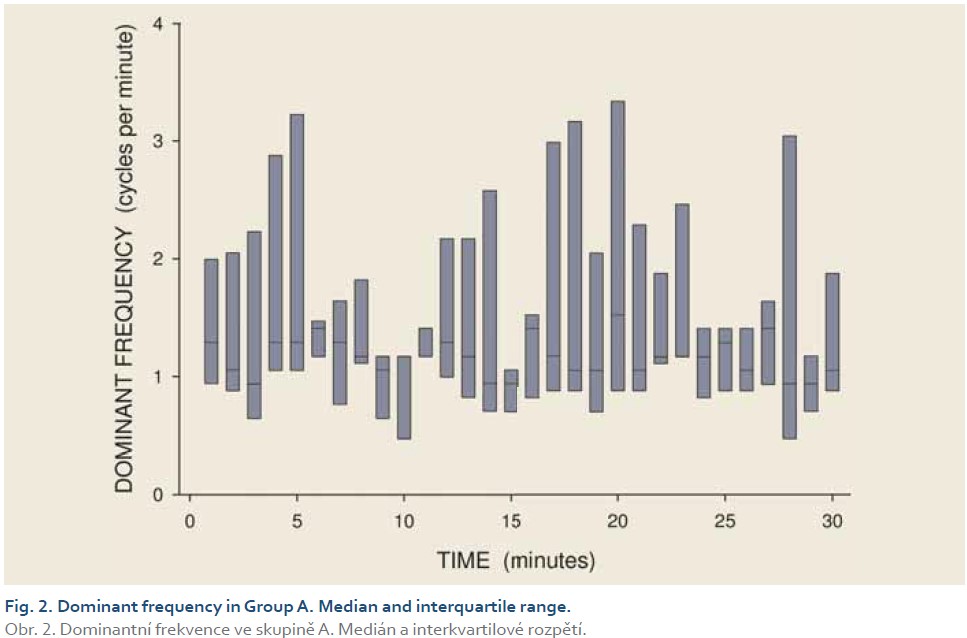
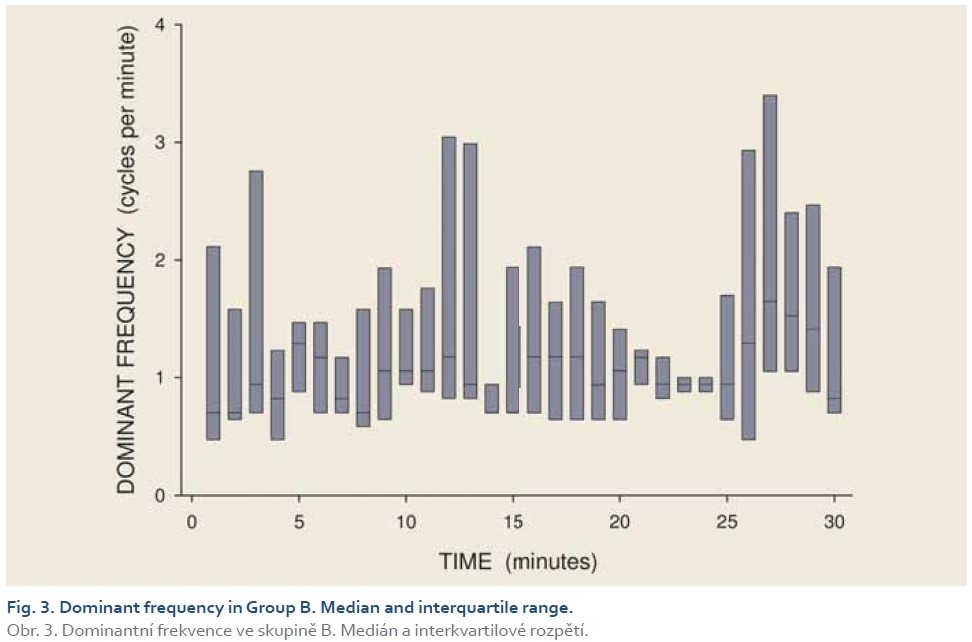
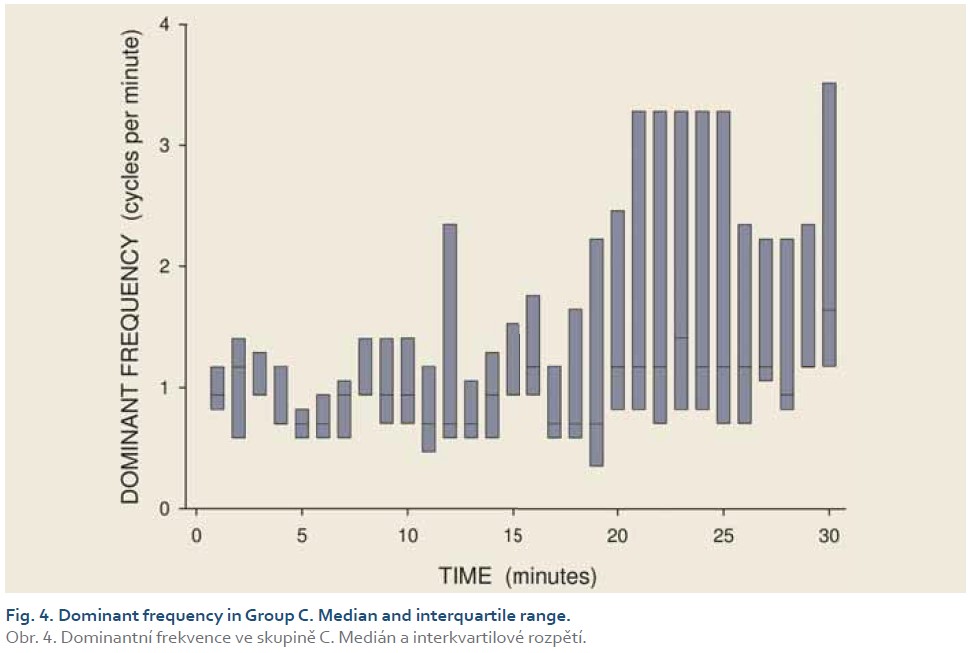
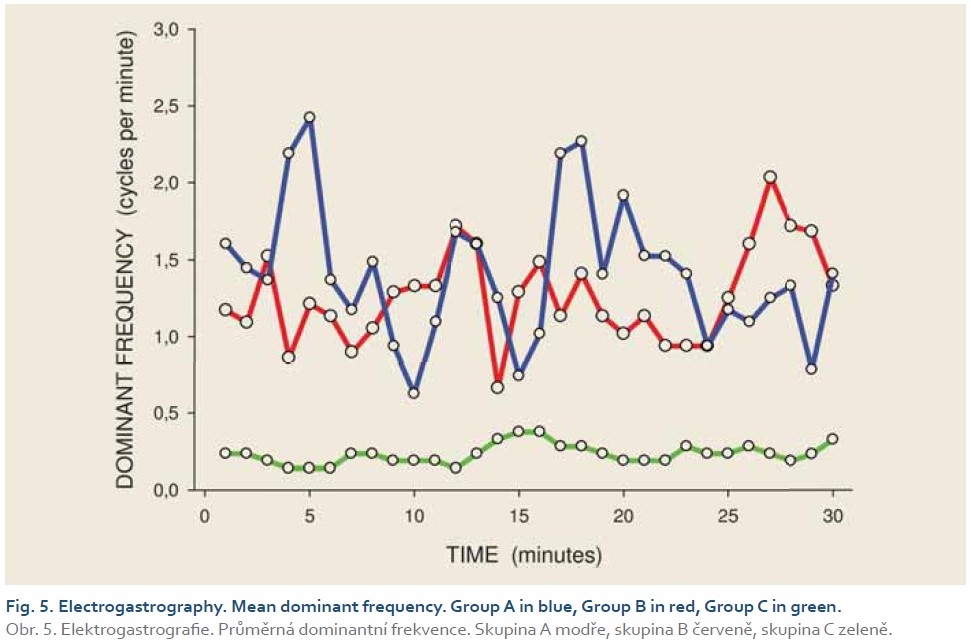
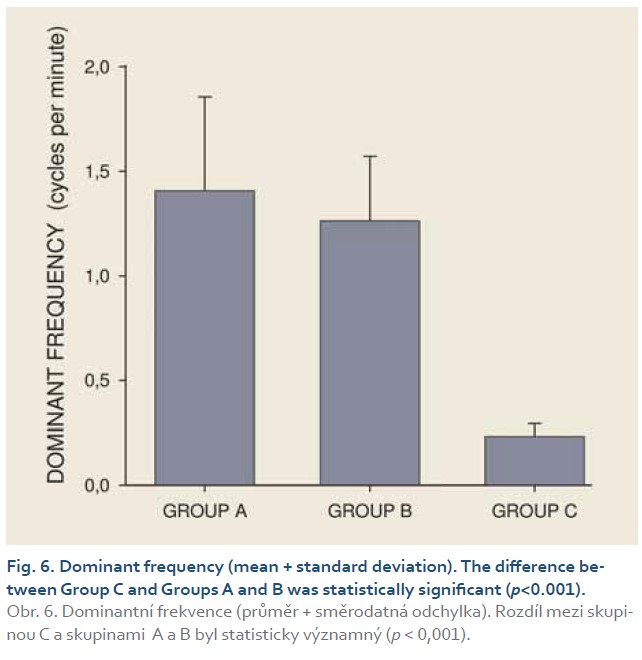
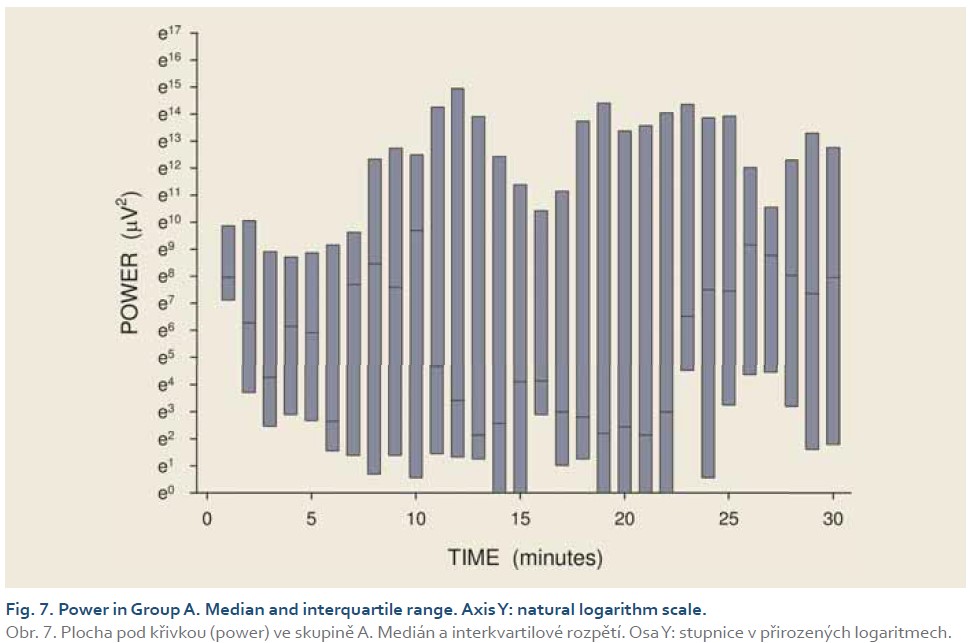
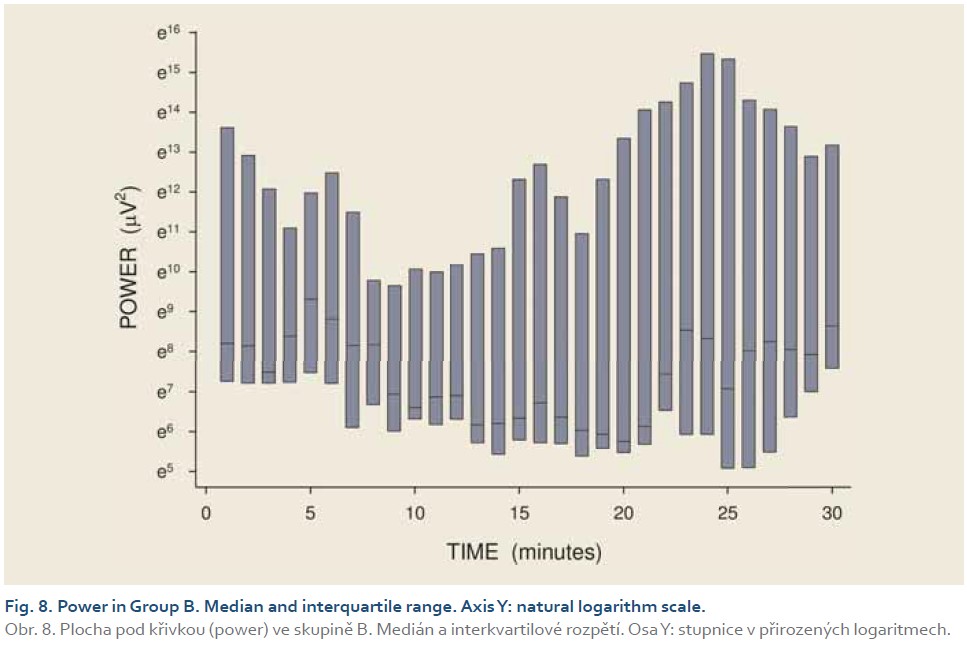
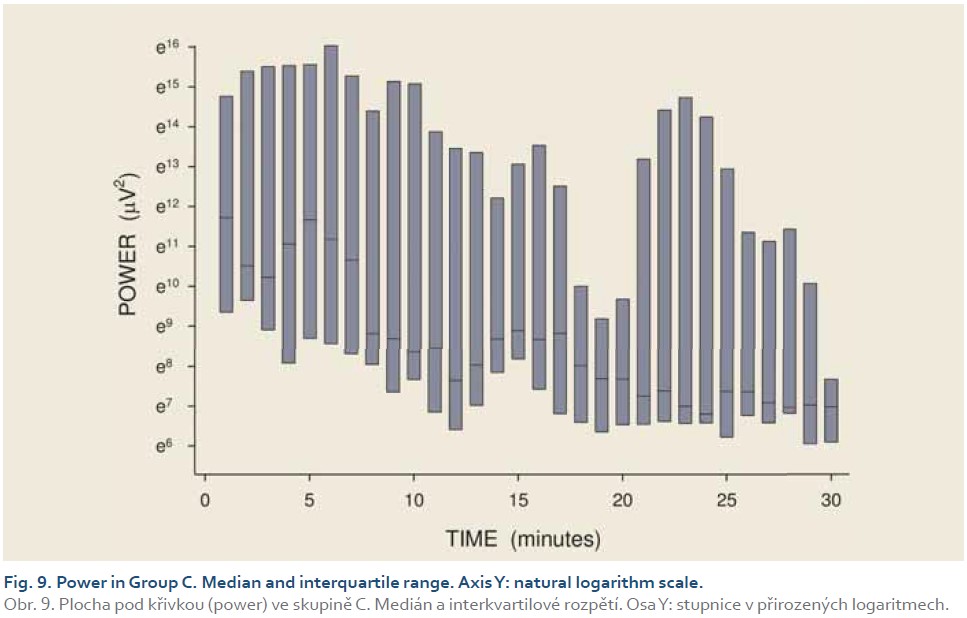
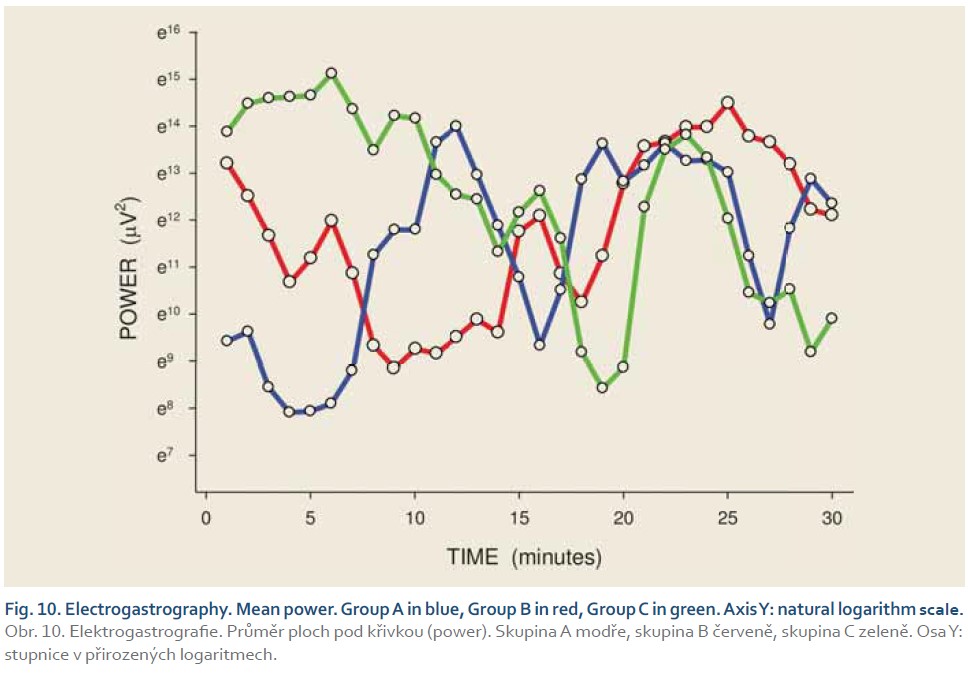
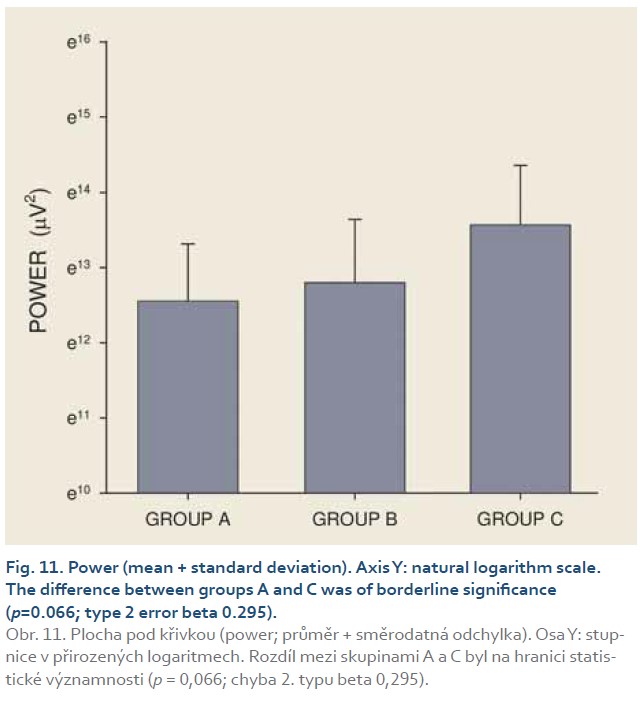
DFs were (mean ± standard deviation): 1.4±0.4 (Group A), 1.3±0.3 (Group B) and 0.2±0.1 cycles per min. (Group C). The differences between group C and groups A and B were statistically significant (p<0.001). Median power (IQR) was 0.13 (0.02–0.44; Group A), 0.13 (0.03–0.54; Group B) and 0.30 V2 (0.07–1.44; Group C). The difference between groups A and C was of borderline significance (p=0.066; type 2 error beta 0.295).
Discussion
Our current study has brought important new insight into the impact of ketamine on porcine gastric myoelectric activity. To the best of our knowledge, this is the first study comparing ketamine and ketamine-free anaesthesia induction in experimental pigs. Surprisingly, ketamine decreased the dominant frequency of gastric slow waves substantially, whereas the impact on EGG power was of borderline significance.
The action of ketamine is mostly dose--dependent [55,56]. In humans, recommended doses for ketamine induction of anaesthesia are 0.5–2 mg/kg (i.v.) and 4–10 mg/kg (i.m.) [50]. Gastrointestinal side effects are reported rarely (nausea, vomiting, increased salivation) [55–58]. Recommended doses of ketamine for veterinary purposes vary considerably. They depend on indications (induction or maintenance of anaesthesia) and they also rely on combination with other drugs and different routes of administration (intravenous, intramuscular, subcutaneous, intranasal, peroral, rectal, intraperitoneal). Recommended doses are substantially distinct between different species (e.g. dogs: 0.5 mg/kg; horses: 2 mg/kg; cats: 11 mg/kg; rats: 80 mg/kg; guinea-pigs: 85 mg/kg) [59]. Recommended doses for ketamine induction of general anaesthesia in experimental pigs are 10–27 mg/kg [46,47,59–62].
The impact of ketamine on gastrointestinal motility has been studied in different species with various designs, different doses, and diverse results [63–67]. Schnoor et al. [61] investigated porcine duodenal motility by means of intraluminal impedance measurement. Ketamine (10 mg/kg i.m.) caused significantly prolonged duration of phase I and shortened phase II of MMC (migrating motor complex) compared to control animals. The authors concluded that the impact of ketamine, even in a single low dose for induction of anaesthesia, should be taken into account in any gastrointestinal motility studies in experimental pigs [61].
The EGG pattern in experimental pigs reveals a substantial inter- and intra-individual variability [40]. Thus, in preclinical research, each animal represents its own actual control, which allows comparison of its own basal EGG (just prior to the experiment) and the immediately-following EGG study recording. A reliable evaluation has been enabled by the assessment of a greater number (thousands) of one-minute EGG intervals [68,69].
We investigated the impact of memantine on porcine EGG in one of our previous trials [53]. Memantine is also an uncompetitive antagonist of NMDA receptors. A single dose of memantine nearly doubled the DF. Although repeated administration of memantine caused marked gastric arrhythmia, the basal DF after single and repeated administration were comparable; however, the DF increase was more prominent in the latter. Basal power was significantly higher after repetitively-administered memantine. Severe gastric arrhythmia and long-lasting low power after repeated administration of memantine might explain possible gastric dysmotility side effects in the chronic use of memantine. We used ketamine as an induction of anaesthesia in that study, and hence a possible gastric myoelectric effect of ketamine might have influenced the basal EGG recording. However, the impact of memantine administration remained unaffected by ketamine use [53]. The different EGG effects of the two NMDA-receptor modulators in these two trials can be explained by the distinct mechanism of their action [70–72].
We are aware of possible limits of our current study. We did not design the project as a cross-over trial. We were not able to evaluate intra-individual variability of experimental animals. Last but not least, we did not use higher and/or repeated doses of ketamine.
Conclusions
Ketamine, administered even at a single intramuscular dose, affected myoelectric function of the porcine stomach. Therefore, it should be avoided in gastrointestinal motility studies in experimental pigs.
Acknowledgements
The authors are very grateful to Ian McColl, MD, PhD, for assistance with the manuscript. The authors are much obliged to Lenka Lacková for her excellent technical cooperation.
ORCID authors
J. Bureš ORCID 0000-0003-0326-117X,
J. Květina ORCID 0000-0002-7115-0247,
V. Radochová ORCID 0000-0002-1477-5451,
M. Zavoral ORCID 0000-0001-7883-7431,
Š. Suchánek ORCID 0000-0003-3659-0252,
S. Rejchrt ORCID 0000-0001-5166-9503,
M. Vališ ORCID 0000-0002-4264-0554,
J. Žďárová Karasová ORCID 0000-0003-0891-9591,
D. Kohoutová ORCID 0000-0001-6937-309X.
Submitted/Doručeno: 10. 7. 202
Accepted/Přijato: 12. 7. 2021
Prof. Jan Bureš, MD, PhD, FCMA
Institute of Gastrointestinal Oncology
Military University Hospital
U Vojenské nemocnice 1200
169 02 Praha 6
bures.jan@uvn.cz
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Said H (ed). Physiology of the Gastrointestinal Tract. 6th ed. London: Academic Press 2018.
2. Gürlich R, Maruna P, Frasko R. Transcutaneous electrogastrography in the perioperative period in patients undergoing laparoscopic cholecystectomy and laparoscopic non-adjustable gastric banding. Obes Surg 2003; 13(5): 714–720. doi: 10.1381/096089203322509273.
3. Pozler O, Neumann D, Vorisek V et al. Development of gastric emptying in premature infants. Use of the 13C-octanoic acid breath test. Nutrition 2003; 19(7–8): 593–596. doi: 10.1016/S0899-9007(03)00064-9.
4. Sýkora J, Malán A, Záhlava J et al. Gastric emptying of solids in children with H. pylori-positive and H. pylori-negative non-ulcer dyspepsia. J Pediatr Gastroenterol Nutr 2004; 39(3): 246–252. doi: 10.1097/00005176-200409000-00004.
5. Bureš J, Kopáčová M, Voříšek V et al. Examination of gastric emptying rate by means of 13C-octanoic acid breath test. Methods of the test for adults and results of the investigation of healthy volunteers [in Czech]. Cas Lek ces 2005; 144(Suppl 3): 18–22.
6. Kojecky V, Bernatek J, Horowitz M et al. Prevalence and determinants of delayed gastric emptying in hospitalised Type 2 diabetic patients. World J Gastroenterol 2008; 14(10): 1564–1569. doi: 10.3748/wjg.14.1564.
7. Frasko R, Maruna P, Gurlich R, Trca S. Transcutaneous electrogastrography in patients with ileus. Relations to interleukin-1beta, interleukin-6, procalcitonin and C-reactive protein. Eur Surg Res 2008; 41(2): 197–202. doi: 10.1159/000134918.
8. Maruna P, Frasko R, Lindner J. Disturbances of gastric electrical control activity after laparotomic cholecystectomy are related to interleukin-6 concentrations. Eur Surg Res 2009; 43(4): 317–324. doi: 10.1159/000235569.
9. O‘Grady G, Angeli TR, Du P et al. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology 2012; 143(3): 589–598.e3. doi: 10.1053/j.gastro.2012.05.036.
10. Michalsky D, Dvorak P, Belacek J et al. Radical resection of the pyloric antrum and its effect on gastric emptying after sleeve gastrectomy. Obes Surg 2013; 23(4): 567–573. doi: 10.1007/s11695-012-0850-6.
11. O‘Grady G, Abell TL. Gastric arrhythmias in gastroparesis: low- and high-resolution mapping of gastric electrical activity. Gastroenterol Clin North Am 2015; 44(1): 169–184. doi: 10.1016/j.gtc.2014.11.013.
12. Carlson DA, Kahrilas PJ, Lin Z et al. Evaluation of Esophageal Motility Utilizing the Functional Lumen Imaging Probe. Am J Gastroenterol 2016; 111(12): 1726–1735.
13. Hajer J, Novák M. Development of an Autonomous Endoscopically Implantable Submucosal Microdevice Capable of Neurostimulation in the Gastrointestinal Tract. Gastroenterol Res Pract 2017; 2017: 8098067. doi: 10.1155/ 2017/8098067.
14. Gharibans AA, Coleman TP, Mousa H et al. Spatial Patterns From High-Resolution Electrogastrography Correlate With Severity of Symptoms in Patients With Functional Dyspepsia and Gastroparesis. Clin Gastroenterol Hepatol 2019; 17(13): 2668–2677. doi: 10.1016/j.cgh.2019.04.039.
15. Sangnes DA, Søfteland E, Bekkelund M et al. Wireless motility capsule compared with scintigraphy in the assessment of diabetic gastroparesis. Neurogastroenterol Motil 2020; 32(4): e13771. doi: 10.1111/nmo.13771.
16. Weusten BLAM, Barret M, Bredenoord AJ et al. Endoscopic management of gastrointestinal motility disorders – part 1: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020; 52(6): 498–515. doi: 10.1055/a-1160-5549.
17. Balihar K, Kotyza J, Zdrhova L et al. Characterization of esophageal motor activity, gastroesophageal reflux, and evaluation of prokinetic effectiveness in mechanically ventilated critically ill patients: a high-resolution impedance manometry study. Crit Care 2021; 25(1): 54. doi: 10.1186/s13054-021-03479-8.
18. Carson DA, O‘Grady G, Du P et al. Body surface mapping of the stomach: New directions for clinically evaluating gastric electrical activity. Neurogastroenterol Motil 2021; 33(3): e14048. doi: 10.1111/nmo.14048.
19. O‘Grady G, Gharibans A, Calder S et al. Retrograde slow-wave activation: a missing link in gastric dysfunction? Neurogastroenterol Motil 2021; 33(4): e14112. doi: 10.1111/nmo.14112.
20. Martinek R, Ladrova M, Sidikova M et al. Advanced Bioelectrical Signal Processing Methods: Past, Present, and Future Approach – Part III: Other Biosignals. Sensors (Basel) 2021; 21(18): 6064. doi: 10.3390/s21186064.
21. McCallum RW, Parkman H, Clarke J et al. Gastroparesis. Pathophysiology, Clinical Presentation, Diagnosis and Treatment. London: Academic Press 2020.
22. Kamiya T, Fukuta H, Hagiwara H et al. Disturbed gastric motility in patients with long-standing diabetes mellitus. J Smooth Muscle Res 2022; 58(0): 1–10. doi: 10.1540/jsmr.58.1.
23. Martinek J, Hustak R, Mares J et al. Endoscopic pyloromyotomy for the treatment of severe and refractory gastroparesis: a pilot, randomised, sham-controlled trial. Gut 2022; 326904. doi: 10.1136/gutjnl-2022-326904.
24. Chen JZ, McCallum RW (eds). Electrogastrography. Principles and Applications. New York: Raven Press 1994.
25. Koch KL, Stern RM. Handbook of Electrogastrography. Oxford: Oxford University Press 2003. doi: 10.1093/oso/9780195147889.001.0001.
26. Parkman HP, Hasler WL, Barnett JL et al. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil 2003; 15(2): 89–102. doi: 10.1046/j.1365-2982.2003.00396.x.
27. Bureš J, Kopáčová M, Voříšek V et al. Correlation of electrogastrography and gastric emptying rate estimated by 13C-octanoic acid breath test in healthy volunteers. Folia Gastroenterol Hepatol 2007; 5(1): 5–11.
28. Bures J, Kabelác K, Kopácová M et al. Electrogastrography in patients with Roux-en-Y reconstruction after previous Billroth gastrectomy. Hepato-Gastroenterology 2008; 55(85): 1492–1496.
29. Alvarez WC, Mahoney LJ. Action currents in stomach and intestine. Am J Physiol 1922; 58(3): 476–493. doi: 10.1152/ajplegacy.1922.58.3.476.
30. Mintchev MP, Otto SJ, Bowes KL. Electrogastrography can recognize gastric electrical uncoupling in dogs. Gastroenterology 1997; 112(6): 2006–2011. doi: 10.1053/gast.1997.v112.pm9178693.
31. Andreis U, Américo MF, Corá LA et al. Gastric motility evaluated by electrogastrography and alternating current biosusceptometry in dogs. Physiol Meas 2008; 29(9): 1023–1031. doi: 10.1088/0967-3334/29/9/002.
32. Koenig JB, Martin CEW, Dobson H et al. Use of multichannel electrogastrography for noninvasive assessment of gastric myoelectrical activity in dogs. Am J Vet Res 2009; 70(1): 11–15. doi: 10.2460/ajvr.70.1.11.
33. Květina J, Edakkanambeth Varayil J, Ali SM et al. Preclinical electrogastrography in experimental pigs. Interdiscip Toxicol 2010; 3(2): 53–58. doi: 10.2478/v10102-010-0011-5.
34. Bures J, Kvetina J, Pavlik M et al. Impact of paraoxon followed by acetylcholinesterase reactivator HI-6 on gastric myoelectric activity in experimental pigs. Neuro Endocrinol Lett 2013; 34(2): 79–83.
35. Květina J, Tachecí I, Pavlík M et al. Use of electrogastrography in preclinical studies of cholinergic and anticholinergic agents in experimental pigs. Physiol Res 2015; 64(5): S647–S652. doi: 10.33549/physiolres.933227.
36. Bureš J, Jun D, Hrabinová M et al. Impact of tacrine and 7-methoxytacrine on gastric myoelectrical activity assessed using electrogastrography in experimental pigs. Neuro Endocrinol Lett 2015; 36(1): 150–155.
37. Poscente MD, Mintchev MP. Enhanced electrogastrography: A realistic way to salvage a promise that was never kept? World J Gastroenterol 2017; 23(25): 4517–4528. doi: 10.3748/wjg.v23.i25.4517.
38. Dallagnol DJR, Corá LA, Gama LA et al. Gastrointestinal Side Effects of Triple Immunosuppressive Therapy Evaluated by AC Biosusceptometry and Electrogastrography in Rats. Endocr Metab Immune Disord Drug Targets 2020; 20(9): 1494–1503. doi: 10.2174/1871530320666200505111456.
39. Sukasem A, Calder S, Angeli-Gordon TR et al. In vivo experimental validation of detection of gastric slow waves using a flexible multichannel electrogastrography sensor linear array. Biomed Eng Online 2022; 21(1): 43. doi: 10.1186/s12938-022-01010-w.
40. Bures J, Kvetina J, Tacheci I et al. The effect of different doses of atropine on gastric myoelectrical activity in fasting experimental pigs. J Appl Biomed 2015; 13(4): 273–277. doi: 10.1016/ j.jab.2015.04.004.
41. Bureš J, Tachecí I, Květina J et al. Experimental electrogastrography [in Czech]. Gastroent Hepatol 2014; 68(3): 237–242.
42. Suenderhauf C, Parrott N. A physiologically based pharmacokinetic model of the minipig: data compilation and model implementation. Pharm Res 2013; 30(1): 1–15. doi: 10.1007/s11095-012-0911-5.
43. Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 1995; 16(5): 351–380. doi: 10.1002/bdd.2510160502.
44. Gonzalez LM, Moeser AJ, Blikslager AT. Porcine models of digestive disease: the future of large animal translational research. Transl Res 2015; 166(1): 12–27. doi: 10.1016/ j.trsl.2015.01.004.
45. Tveden-Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol 2018; 123(3): 233–235. doi: 10.1111/bcpt.13059.
46. Boschert K, Flecknell PA, Fosse RT et al. Ketamine and its use in the pig. Recommendations of the Consensus meeting on Ketamine Anaesthesia in Pigs, Bergen 1994. Ketamine Consensus Working Group. Lab Anim 1996; 30(3): 209–219. doi: 10.1258/002367796780684863.
47. Pehböck D, Dietrich H, Klima G et al. Anesthesia in swine: optimizing a laboratory model to optimize translational research. Anaesthesist 2015; 64(1): 65–70. doi: 10.1007/s00101-014-2371-2.
48. Nowacka A, Borczyk M. Ketamine applications beyond anesthesia – A literature review. Eur J Pharmacol 2019; 860: 172547. doi: 10.1016/j.ejphar.2019.172547.
49. Carlsen MF, Christoffersen BØ, Lindgaard R et al. Implantation of telemetric blood pressure transmitters in Göttingen Minipigs: Validation of 24-h systemic blood pressure and heart rate monitoring and influence of anaesthesia. J Pharmacol Toxicol Methods 2022; 115: 107168. doi: 10.1016/j.vascn.2022.107168.
50. Zanos P, Moaddel R, Morris PJ et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev 2018; 70(3): 621–660. doi: 10.1124/ pr.117.015198.
51. Kurdi MS, Theerth KA, Deva RS. Ketamine: Current applications in anesthesia, pain, and critical care. Anesth Essays Res 2014; 8(3): 283–290. doi: 10.4103/0259-1162.143110.
52. Persson J. Wherefore ketamine? Curr Opin Anaesthesiol 2010; 23(4): 455–460. doi: 10.1097/ACO.0b013e32833b49b3.
53. Bures J, Kvetina J, Radochova V et al. The pharmacokinetic parameters and the effect of a single and repeated doses of memantine on gastric myoelectric activity in experimental pigs. PLoS One 2020; 15(1): e0227781. doi: 10.1371/journal.pone.0227781.
54. Explanatory Report on the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (ETS 123). Strasbourg: Council of Europe, 1986.
55. Wolff K, Winstock AR. Ketamine: from medicine to misuse. CNS Drugs 2006; 20(3): 199–218. doi: 10.2165/00023210-200620030-00003.
56. Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol 2008; (182): 313–333. doi: 10.1007/978-3- 540-74806-9_15.
57. White PF, Way WL, Trevor AJ. Ketamine: its pharmacology and therapeutic uses. Anesthesiology 1982; 56(2): 119–136. doi: 10.1097/ 000 00542-198202000-00007.
58. Green SM, Nakamura R, Johnson NE. Ketamine sedation for pediatric procedures: Part 1, A prospective series. Ann Emerg Med 1990; 19(9): 1024–1032. doi: 10.1016/s0196-0644(05)82568-5.
59. Cicero L, Fazzotta S, Palumbo VD et al. Anesthesia protocols in laboratory animals used for scientific purposes. Acta Biomed 2018; 89(3): 337–342. doi: 10.23750/abm.v89i3.5824.
60. Schnoor J, Bartz S, Klosterhalfen B et al. A long-term porcine model for measurement of gastrointestinal motility. Lab Anim 2003; 37(2): 145–154. doi: 10.1258/00236770360563796.
61. Schnoor J, Unger JK, Kochs B et al. Effects of a single dose of ketamine on duodenal motility activity in pigs. Can Vet J 2005; 46(2): 147–152.
62. Linkenhoker JR, Burkholder TH, Linton CG et al. Effective and safe anesthesia for Yorkshire and Yucatan swine with and without cardiovascular injury and intervention. J Am Assoc Lab Anim Sci 2010; 49(3): 344–351.
63. Healy TE, Foster GE, Evans DF et al. Effect of some i.v. anaesthetic agents on canine gastrointestinal motility. Br J Anaesth 1981; 53(3): 229–233. doi: 10.1093/bja/53.3.229.
64. Shinozaki H, Gotoh Y, Ishida M. Selective N-methyl-D-aspartate (NMDA) antagonists increase gastric motility in the rat. Neurosci Lett 1990; 113(1): 56–61. doi: 10.1016/0304-3940(90)90494-t.
65. Kounenis G, Koutsoviti-Papadopoulou M, Elezoglou V. Ketamine may modify intestinal motility by acting at GABAA-receptor complex; an in vitro study on the guinea pig intestine. Pharmacol Res 1995; 31(6): 337–340. doi: 10.1016/1043-6618(95)80086-7.
66. Fass J, Bares R, Hermsdorf V et al. Effects of intravenous ketamine on gastrointestinal motility in the dog. Intensive Care Med 1995; 21(7): 584–589.
67. Elfenbein JR, Robertson SA, Corser AA et al. Systemic effects of a prolonged continuous infusion of ketamine in healthy horses. J Vet Intern Med 2011; 25(5): 1134–1137.
68. Bures J, Tacheci I, Kvetina J et al. The Impact of Dextran Sodium Sulfate-Induced Gastrointestinal Injury on the Pharmacokinetic Parameters of Donepezil and Its Active Metabolite 6-O-desmethyldonepezil, and Gastric Myoelectric Activity in Experimental Pigs. Molecules 2021; 26(8): 2160. doi: 10.3390/molecules26082160.
69. Bures J, Tacheci I, Kvetina J et al. Dextran Sodium Sulphate-Induced Gastrointestinal Injury Further Aggravates the Impact of Galantamine on the Gastric Myoelectric Activity in Experimental Pigs. Pharmaceuticals (Basel) 2021; 14(6): 590. 10.3390/ph14060590.
70. Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A 2014; 111(23): 8649–8654. doi: 10.1073/pnas.1323920111.
71. Johnson JW, Glasgow NG, Povysheva NV. Recent insights into the mode of action of memantine and ketamine. Curr Opin Pharmacol 2015; 20: 54–63. doi: 10.1016/j.coph.2014.11.006.
72. Glasgow NG, Povysheva NV, Azofeifa AM et al. Memantine and Ketamine Differentially Alter NMDA Receptor Desensitization. J Neurosci 2017; 37(40): 9686–9704. doi: 10.1523/JNEUROSCI. 1173-17.2017.