Biosimilar infliximab CT-P13 treatment in patients with inflammatory bowel diseases – a one-year, single-centre retrospective study
Tibor Hlavatý Orcid.org 1, Anna Krajčovičová Orcid.org 1, Igor Šturdík Orcid.org 1, Juraj Letkovský2,3, Tomáš Koller Orcid.org 4, Jozef Tóth Orcid.org 4,3, Martin Huorka Orcid.org 4
+ Affiliation
Summary
Background and Aims: CT-P13 is the first approved infliximab (IFX) biosimilar drug. It has been proven to be equivalent to infliximab in several rheumatoid disorders; however, limited efficacy data are available for its use in inflammatory bowel disease. We evaluated our 1-year clinical data with CT-P13 to assess its efficacy and safety in inflammatory bowel disease patients. Methods: This retrospective cohort study included all consecutive Crohn’s disease (CD) or ulcerative colitis (UC) patients treated between March 2014 and April 2015. Clinical remission was evaluated at 14 weeks after the first induction treatment. Sustained clinical responses during CT-P13 maintenance treatments were assessed every eight weeks, both in CT-P13-induced patients and those switching from IFX. Results: The cohort included 25 inflammatory bowel disease patients (19 CD, six UC). There was a median of five CT-P13 administrations per patient (range: 2–9) and a total of 128 total infusions. Thirteen patients (nine CD, four UC) underwent CT-P13 induction, and 84% achieved clinical remission (7/9 CD, 4/4 UC) by week 14. A sustained clinical response was observed in 85% of patients (3/3 CD, 3/4 UC) at week 30. Twelve patients (10 CD, two UC) switched from infliximab, and 100% showed a sustained clinical response at week 24, 87.5% (6/7 CD, 1/1 UC) at week 32, and 75% (5/7 CD, 1/1 UC) at week 48. Four CD patients discontinued CT-P13 therapy because they experienced infusion reactions or psoriasiform skin rashes, or because they showed a loss of response. Conclusion: Our data indicate that CT-P13 is comparable to IFX in terms of effectiveness and safety.
Keywords
infliximab, CT-P13, Crohn’s disease, biosimilars, retrospective study, therapy, ulcerative colitisIntroduction
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are idiopathic, chronic, relapsing inflammatory con-ditions of the gastrointestinal tract. Inadequate treatment of moderate--to-severe IBD can result in disease progression, leading to intestinal and extraintestinal complications, major abdominal surgery, and disability [1].
Infliximab (IFX) was the first FDA- -approved monoclonal antibody targeting tumor necrosis factor α (TNF-α). IFX treatment has shown significant success in patients with moderate- -to-severe luminal and fistulising CD, moderate-to-severe UC, and several extraintestinal IBD complications in both children and adults [1–6]. However, IFX is costly, which limits its affordability [7].
Biosimilars are copies of biologic medicinal products. They have similar physicochemical characteristics, biological activity, clinical efficacy, and safety. CT-P13 was the first IFX biosimilar approved for use in adults and children with CD and UC [8]. CT-P13 costs less than IFX, which increases the accessibility of this biological therapy for low-income patients [9,10]. In fact, economic modelling analyses predicted that CT-P13 would yield significant cost savings compared with IFX [11,12].
CT-P13 and IFX have identical amino- acid sequences, and are produced using the same human/mouse cell hybrid clone technology [13,14]. However, because biosimilars are manufactured in vitro, they possess subtle structural heterogeneity that could affect their efficacy or immunogenicity [15,16]. Therefore, there is a critical need for reports describing the clinical results of newly introduced biosimilars, especially those describing treatments for indications that have not been formally studied.
Thus far, two head-to-head clinical trials have been performed comparing CT-P13 and infliximab for rheumatologic conditions. The first was a Phase I trial called PLANETAS that studied 250 ankylosing spondylitis patients [17]. The second was a Phase III trial called PLANETRA [14]. This study enrolled 606 patients with rheumatoid arthritis who still had active disease despite receiving methotrexate treatment [14,17]. Both trials showed that CT-P13 was comparable to IFX in terms of efficacy and safety, and the treatment was generally well tolerated. Reports regarding CT-P13 in the IBD field are limited. Consequently, questions have been raised about its efficacy, safety, and interchangeability with IFX in IBD [10,18,19].
In Slovakia, CT-P13 first became available in February 2014. Our tertiary care hospital implemented CT-P13 treatment for IBD patients soon after. The aim of our study was to retrospectively assess CT-P13’s efficacy and safety in IBD patients over the first 12 months after the drug became available.
Materials and Methods
Study design and patient selectionThis retrospective, single-centre cohort study included all consecutive adult (≥ 18 years old) patients with CD or UC who were treated with CT-P13 in the IBD centre of the Department of Internal Medicine, University Hospital Bratislava, Ruzinov, Slovakia between March 2014 and April 2015. This study was reviewed and approved by the Ethics Committee of the University Hospital Bratislava. All data in the research database were anonymised by replacing each subject’s personal identification data with a unique research ID number. All subjects provided written, informed consent approving use of their clinical and laboratory data for analyses.
CT-P13 was used for induction or maintenance treatment in patients with moderate-to-severe IBD. The study group on maintenance therapy also included patients with IFX who were switched to CT-P13. The induction regime consisted of three 5 mg/kg CT-P13 infusions administered intra- venously at weeks 0, 2 and 6. All patients who achieved remission by week 14 were converted to CT-P13 maintenance treatment. Maintenance treatment consisted of intravenous 5 mg/kg CT-P13 infusions administered every eight weeks; however, this regime was increased to an infusion every four weeks if the physician noted a loss of response (LOR). Remission and LOR are defined in the CT-P13 efficacy and safety evaluation section. The inclusion criteria for the whole cohort of the present study were as follows: if the patient had received at least three CT-P13 infusions prior to the end of the study period, or if treatment had to be terminated due to an adverse event. The patients received concomitant treatment with mesalazine, predni- sone, or azathioprine if the gastroenterologist deemed it clinically necessary.
Clinicopathological variables
The clinical and laboratory data of all eligible patients at baseline and follow-up were extracted from the patients’ electronic health records in the hospital information system. The baseline data collected included demographic and clinical characteristics such as age, sex, disease dura- tion, disease location, disease behav- iour defined by the Montreal classi- fication [20], use of concomitant medication, use of previous anti- -TNF-α therapy, and history of major IBD-related abdominal surgery. For patients who switched from IFX maintenance therapy, the duration and dosing of the prior IFX treatments were also retrieved.
The following data were collected at baseline and every subsequent follow-up visit: clinical activity, clinical assessment of perianal fistulas, disease--related quality of life (QoL), use of concomitant immunosuppressive medication, and C-reactive protein (CRP) levels, when available. CD and UC clinical activity was assessed at every visit using the Harvey-Bradshaw index (HBI) and the partial Mayo score (pMayo), respectively [21–23]. The pMayo score was used instead of the total Mayo score because not all patients underwent colonoscopy during CT-P13 therapy. The disease- -related QoL was assessed at each visit using the short Inflammatory Bowel Diseases Questionnaire (sIBDQ) [24].
CT-P13 efficacy and safety evaluation
Clinical remission of CD patients was defined as having an HBI score < 5 and no active fistulas (i.e. closure of all draining fistulae, no perianal pain, and no signs of fistula sepsis) [4]. Clinical remission of UC patients was defined as a pMayo score < 2 [25]. Patients who achieved clinical remission with CT-P13 induction progressed to CT-P13 maintenance therapy. Both the induction patients and those patients who switched to CT-P13 from IFX maintenance therapy were evaluated for sustained clinical response every eight weeks. Sustained clinical response for CD patients was defined as follows: the HBI score did not increase by more than two points, any fistulas remained inactive, and no adverse events or LOR leading to CT-P13 discontinuation occurred. Sustained clinical response for UC patients was defined as follows: the pMayo score did not increase by more than one point and no adverse events or LOR leading to CT-P13 discontinuation occurred.
Adverse events of special interest
Adverse events of special interest were collected at each follow-up visit. These adverse events included severe or opportunistic infections, acute or delayed hypersensitivity reactions, treatment discontinuation due to side effects, and IBD-related hospital admission or surgery.
Statistical analyses
Statistical tests were performed using SPSS 19.0 software (IBM SPSS Inc., Chicago, Illinois, USA). Graphs were plotted using Analyse-it in Microsoft Excel (Analyse-it Software Ltd, Leeds, UK). Nominal and ordinal variables, such as clinical characteristics, clinical remission rate, sustained clinical response rate, and adverse event rates, are presented as frequency and percentage. Continuous variables, including age, duration of treatment, HBI score, pMayo score, sIBDQ score, and CRP levels, were tested for normal distribution using the Kolmogorov--Smirnov test. All variables except sIBDQ scores had non-normal distri-butions and are presented as median, quartiles, and range. The statistical significance of the change in HBI score, pMayo score, and CRP levels between baseline and follow-up was tested using the Wilcoxon rank sum test. sIBDQ was reported as mean ± standard deviation, and the changes between baseline and follow-up were tested with a paired Student’s t-test. A p value < 0.05 was considered statistically significant.
Results
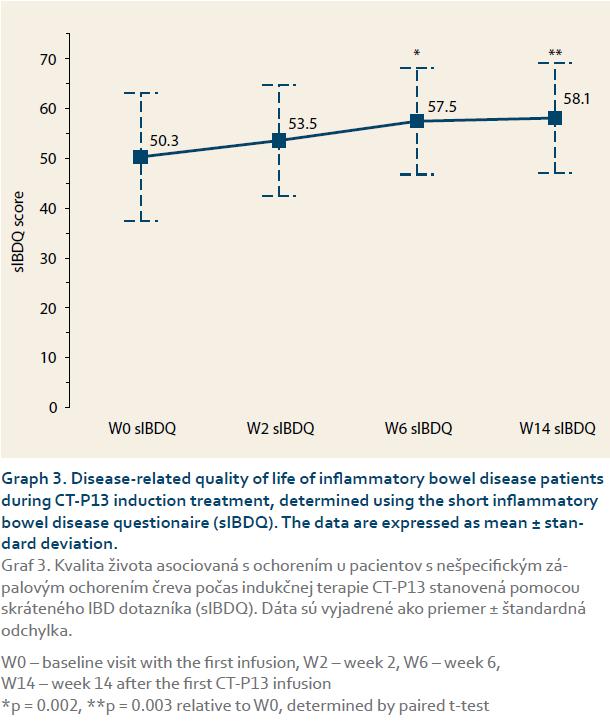
Study population characteristicsIn total, 25 IBD patients treated with CT-P13 during the study period met the inclusion criteria. Out of these, 19 had CD, and six had UC. The demographic and clinical characteristics of the cohort are presented in table 1. Regarding treatments, 13 patients received CT-P13 induction therapy and 12 switched from IFX to CT-P13 maintenance therapy. In all maintenance therapy cases, the reason for switching was the reduced treatment cost. Overall, 25 patients received 128 CT-P13 infusions total, and there was a median of five infusions per patient (range 2–9).
CT-P13 induction treatment effectiveness
In total, 13 IBD patients underwent the six-week CT-P13 induction treatment. Of those, nine had CD and four had UC. The treatment indications for CD patients were luminal disease (n = 6) or combined luminal/perianal fistulising disease (n = 3). The treatment indications for UC patients were moderate disease activity (n = 3) or mild activity complicated with severe nodular erythema (n = 1). At weeks 2 and 6, the clinical remission rates of all 13 induction therapy patients were 77% (10/13). At week 14, the clinical remission rate was 84% (11/13) for all induction patients, 78% for the CD patients (7/9), and 100% for the UC patients (4/4). All three CD patients with fistulas experienced complete lesion closure. Out of the 13 induction patients, seven (three with CD, four with UC) received concomitant corticosteroid treatment at baseline (15–30 mg prednisone). All of these patients discontinued steroid treatment within 10 weeks after the first CT-P13 infusion. CT-P13 treatment was discontinued after the second infusion for two CD patients due to acute hypersensitivity infusion reactions (described in section Safety and adverse events of special interest).
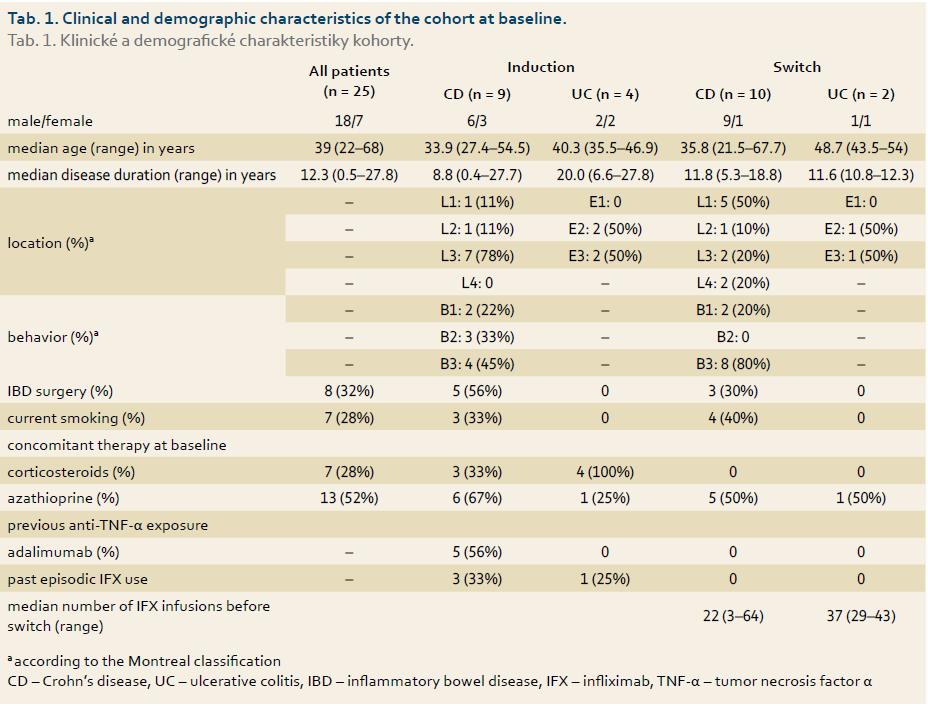
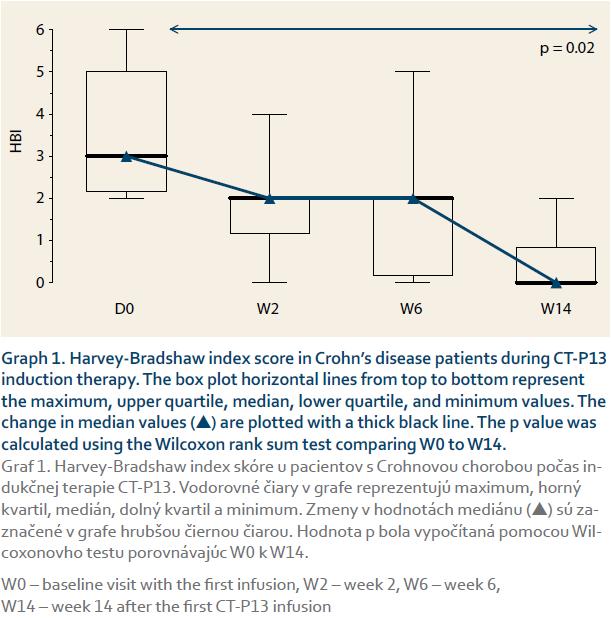
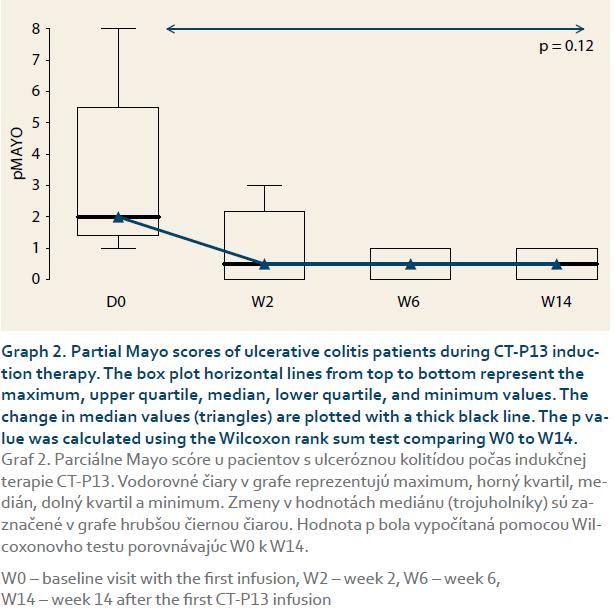
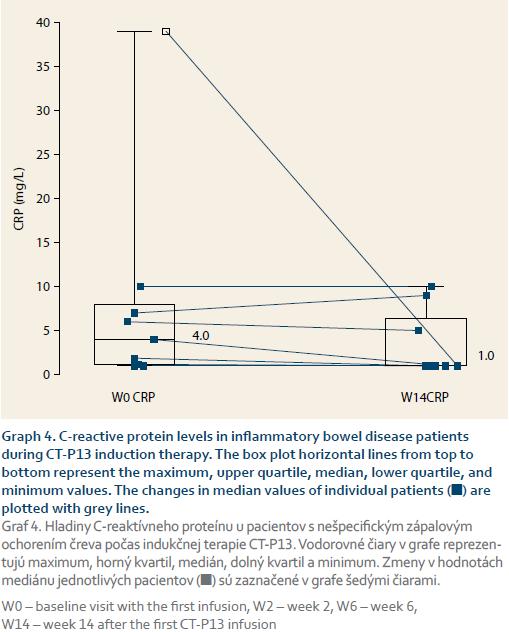
The CD patients’ median HBI scores dropped significantly from 3 at baseline to 0 at week 14 (p = 0.02, graph 1). Similarly, the UC patients’ median pMayo scores dropped from 2 at baseline to 0.5 at week 14 (p = 0.12 between week 0 and 14), (graph 2). The mean sIBDQ scores of the induction group improved significantly from 50.3 ± 12.8 at baseline to 57.5 ± 10.7 at week 6 (p = 0.002) and 58.1 ± 11.0 at week 14 (p = 0.003) (graph 3). CRP values were collected for nine induction patients. The median CRP levels dropped from 4.0 mg/L (interquartile range (IQR) 7.4) at baseline to 1.0 mg/L (IQR 4.7) at week 14 (p = 0.13, graph 4).
Maintenance treatment effectiveness following induction therapy
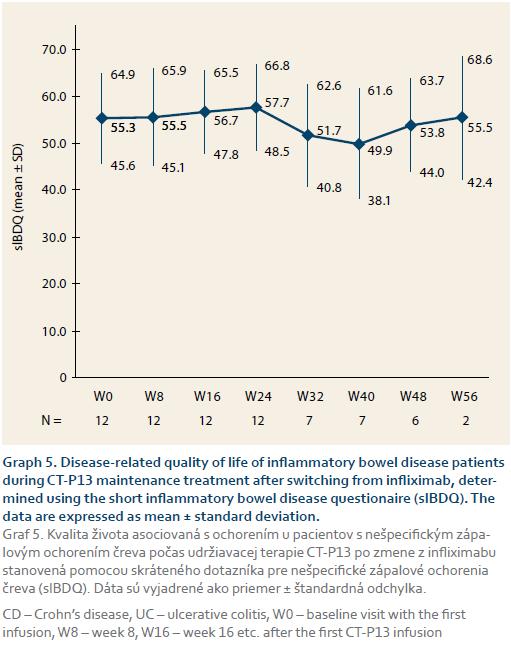
After 14 weeks of CT-P13 induction therapy, patients were switched to maintenance therapy. The median follow-up time for patients who achieved clinical remission was 24 weeks after the first CT-P13 infusion. The patients’ QoL remained stable during follow-up and there were no significant sIBDQ score changes between week 14 (the maintenance therapy baseline) and any of the follow-up visits.
By April 2015, seven of these patients (three CD, four UC) had received at least 30 weeks of follow-up maintenance therapy. At week 30 after the first CT-P13 infusion, sustained clinical response was maintained in six of the seven patients (85% total, 3/3 CD, 3/4 UC). One UC patient discontinued CT-P13 maintenance due to an acute hypersensitivity reaction during the fourth infusion at week 14. Only two patients completed 54 weeks of follow-up. One CD patient experienced an LOR, indicated by perianal fistula reopening. This patient’s dose interval was reduced to four weeks between infusions. By week 54, this patient re-entered clinical remission accompanied by fistula closure. The UC patient remained on the normal maintenance regime throughout the study period, and remission persisted through week 54.
Maintenance treatment effectiveness after switching from infliximab
To evaluate the effectiveness of CT-P13 in patients induced with the originator, IFX, twelve IBD patients (10 CD, two UC) switched from IFX to CT-P13 maintenance therapy. The median maintenance therapy follow-up time for these patients was 44 weeks. Sustained clinical response was maintained in all 12 patients at week 24. At weeks 32 and 40, seven of eight patients (87.5% total, 6/7 CD, 1/1 UC) sustained clinical response. At week 48, six of eight patients (75% total, 5/7 CD, 1/1 UC) remained in remission. The mean sIBDQ scores did not change significantly at any of the follow-up visits, indicating that the patients’ QoL was stable during maintenance therapy (graph 5).
One CD patient showed LOR, and dose intensification was initiated at week 16 after the switch. The patient regained clinical remission, and it was maintained until the end of the study period. Two patients discontinued CT-P13 after the switch. One CD patient developed psoriasiform dermatitis (described in section Safety and adverse events of special interest). Notably, this patient retained clinical remission throughout the CT-P13 treatment period. Another CD patient with a history of extensive fistulising perianal disease experienced an LOR during CT-P13 maintenance therapy. This patient received three IFX infusions prior to the switch. The CT-P13 dose was intensified to administration every four weeks; however, no improvements were ob- served, and CT-P13 treatment was discontinued. This patient was subsequently switched to adalimumab.
Safety and adverse events of special interest
Overall, CT-P13 was well tolerated. None of the patients experienced severe or opportunistic infections, and there were no IBD-related hospital admissions or abdominal surgeries during the follow-up period. As mentioned above, two patients experienced acute hypersensitivity reactions to the second CT-P13 infusion. Both reactions occurred in CD patients receiving CT-P13 induction treatments. Both patients had been treated with IFX episodically prior to CT-P13 treatment.
One patient experienced psoriasiform dermatitis after the fourth CT-P13 infusion. This patient had received 22 IFX infusions prior to switching to CT-P13. The dermatitis resolved itself after CT-P13 discontinuation. The patient was subsequently switched to adalimumab and retained clinical remission.
Discussion
The purpose of this retrospective analysis was to evaluate outcomes for IBD patients treated with the IFX biosimilar CT-P13. Our data showed an 84% clinical remission rate (78% CD, 100% UC) at week 14 after initiating the six-week CT-P13 induction therapy. Furthermore, all seven patients taking corticosteroids at the start of induction therapy discontinued steroid usage within 10 weeks after the first CT-P13 infusion. The general QoL of IBD patients improved significantly, as determined by improvement in mean sIBDQ score. Multiple randomised controlled trials and retrospective cohort studies have evaluated IFX induction therapy efficacy in patients with IBD [2–5,26,27]. For CD, the reported response rates ranged from 48–89%, and the remission rates 4–10 weeks after the first infusion were 30–59% [2–4,26–30]. For UC, two randomised controlled trials of IFX treatment (ACT 1 and 2) showed eight-week clinical response and remission rates of 69% and 39%, resp., for ACT 1, and 65% and 34%, resp., for ACT 2 [5]. Previous cohort studies generally reported higher IFX efficacies in UC, with reported response rates ranging 59–93% and week 2–14 remission rates ranging 24–69% [31–33]. Given our small cohort size and retrospective study design, we cannot directly compare our study to those described above in a quantitative manner. Qualitatively, our observed remission rates are higher than those reported in previous studies. However, this could be due to our evaluation of the remission rate at week 14 (fourth infusion), the number of patients that used concomitant azathioprine at baseline, and also the relatively low median HBI and partial Mayo at the time of the first infusion.
In our study, 85% of patients who entered clinical remission following induction therapy had a sustained clinical response with maintenance therapy at week 30. Moreover, in the patients who switched maintenance treatment from IFX to CT-P13 maintenance therapy, 75% had a sustained clinical response 48 weeks after the switch. Previous studies of IFX maintenance therapy have shown that it is efficacious and well tolerated [5,25,34,35]. In a large cohort of 614 CD patients treated with IFX maintenance therapy for 55 months, 81% of patients were primary responders, and 63.4% of patients retained a sustained benefit by the end of the study period. The yearly drop-out rate was 7.1–10.7% depending on whether IFX treatment was scheduled or episodic. In total, 12.8% and 21.6% had to stop IFX due to side effects and LOR, respectively [36]. Similarly, a retrospective Canadian study on CD patients showed that the sustained response rates at weeks 30 and 54 were 83.2% and 63.6%, respectively [37]. Our data are consistent with those described for IFX, indicating that CT-P13 maintenance therapy for IBD is efficacious and well tolerated.
LOR is a significant consideration in biologic treatments. In our study, LOR was observed in three of the 25 (12%) CT-P13-treated patients, all of which had CD. The first LOR occurred in the induction group at week 46 (1/13, 8%), and the other two occurred 16 and 32 weeks after switching from IFX (2/12, 17%). In all three cases, the CT-P13 dose was escalated by reducing the dosing interval from eight to four weeks. The induction LOR and 16-week IFX-switch LOR patients regained clinical remission within two CT-P13 infusions. However, the 32-week IFX-switch LOR patient had extensive perianal fistulas and did not respond to CT-P13 dose escalation. Previous studies of IFX maintenance therapy have shown that LOR is fairly common long term. An 11-year retrospective study of IFX treatment for CD observed that 57% of patients required a dose change and 8% discontinued treatment due to LOR [30]. Similarly, a 54- -month randomised control trial of IFX maintenance therapy for CD showed that 42% of IFX-treated patients experienced LOR compared with 62% in placebo-treated patients. In that study, 57% of patients reestablished response by increasing the dose from 5 to 10 mg/kg [4]. When considering the observation period differences between our study (one year) and previous studies (4.5–13 years), we expect to see much lower LOR rates for our CT-P13 therapies when compared with those IFX therapies. It is highly likely that a longer study with a larger number of patients will produce LOR rates similar to those seen for IFX therapies.
In our study, CT-P13 therapy had to be discontinued in 12% (3/25) of patients due to adverse events. Reasons for discontinuation included two delayed hypersensitivity reactions and one case of psoriasiform dermatitis. A number of previous IFX studies report delayed hypersensitivity reactions at rates ranging 1–6% [5,25,30,36]. This is in the line with our observation. While it is tempting to state that CT-P13 is relatively safe short term, given the brief observation period and small number of patients, drawing a definitive conclusion is not advisable.
Currently, clinical experience with CT-P13 as an IBD treatment is limited. The first clinical report on CT-P13 treatment for IBD was a single-centre study in South Korea with a 17 patient cohort (8 CD, 9 UC) [38]. Of these, eight underwent CT-P13 induction therapy. Similar to our study, clinical remission was achieved at week 8 after induction in all five UC and two of the three CD patients. The remaining nine patients (four UC, five CD) had previously undergone IFX maintenance therapy and were switched to CT-P13. Therapy was discontinued in one CD patient due to LOR and one UC patient due to arthralgia development. A retrospective, multicentre study, also from Korea, was recently published [39]. This cohort consisted of 110 IBD patients (59 CD, 51 UC). For patients undergoing induction therapy, at week 8 patients exhibited an 85% clinical response rate (29/32 CD, 34/42 UC) and a 58% remission rate (27/32 CD, 16/42 UC). At week 54, patients exhibited a 95% clinical response rate (7/8 CD, 12/12 UC) and a 60% remission rate (6/8CD, 6/12UC). CT-P13 was discontinued in four UC patients due to skin rash, an infusion reaction, leukopenia, and B-viral hepatitis reactivation. In patients who switched from IFX, treatment efficacy was maintained in 92.6% (25/27) of CD patients and 66.7% (6/9) of UC patients. In addition, five patients (two CD, three UC) discontinued CT-P13 due to LOR, one discontinued because they wanted to switch back to IFX, and one discontinued due to adverse events (skin rash and arthralgia).
Jarzębicka et al. recently presented the interim results from a study of CT--P13 induction therapy in 18 children with IBD (six UC, 12 CD) [40,41]. A clinical response was seen in three out of four UC patients and 10 out of 12 CD patients by week 10. Therapy was discontinued in two CD and one UC patients due to LOR. One UC and one CD patient discontinued therapy due to infusion reactions. They also evaluated the short-term results of switching from IFX to CT-P13 in a cohort of 32 pediatric CD patients [42]. Flare-ups were not observed after the first or second CT-P13 infusion. Moreover, serious adverse events and infusion reactions were not observed. In addition, a Hungarian group recently reported similar interim results. A cohort of 90 IBD (57 CD, 33 UC) were administered CT-P13 induction therapy [43]. At six weeks after the first infusion, the Crohn’s Disease Activity Index and pMayo scores in CD and UC patients, respectively, dropped significantly. In total, four infusion reactions occurred, all in patients who had previously received anti-TNF-α therapy. While we cannot draw any strong conclusions due to the preliminary nature of these data, the results are, on the surface, similar to our own. When considering all the available data on CT-P13 treatment for IBD, the results indicate that CT-P13 could be a viable and lower-cost replacement for IFX, especially for anti-TNFα–naïve individuals.
Our study has several limitations. First, it is a retrospective study, which limits the complexity of the data, especially in terms of safety. Second, it is a single-centre study, and the cohort only included 25 patients, which limits statistical power. The study population was also heterogeneous, including both CD and UC patients. Third, there was no control group to compare CT-P13 to IFX. Nevertheless, given the scarcity of data on CT-P13 treatment of IBD to date, our clinical experience in the first 12 months using CT-P13 may be useful.
We can derive several clinical implications from our results. First, CT-P13 induction treatment for IBD seems to be as effective as IFX. Second, the switch from IFX to CT-P13 was largely safe, and patients showed clinical relapse and adverse event rates similar to those reported for IFX. Lastly, CT-P13’s safety profile in IBD patients seems to be similar to that of IFX. However, given the limitations of our study and the two published previously, it is clear that prospective large-scale studies of CT-P13 safety and interchangeability with IFX in IBD patients are needed.
Abbreviations
CD – Crohn’s disease
CRP – C-reactive protein
HBI – Harvey-Bradshaw index
IFX – Infliximab
IBD – Inflammatory bowel disease
IQR – Interquartile range
LOR – Loss of response
pMayo – Partial Mayo Score
sIBDQ – Short Inflammatory Bowel Diseases Questionnaire
TNF-α – Tumor necrosis factor α
UC – Ulcerative colitis
The authors declare they have no potential confl icts of interest concerning drugs,
products, or services used in the study.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“
for bio medical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané
do bio medicínských časopisů.
Submitted/Doručeno: 8. 12. 2015
Accepted/Přijato: 12. 1. 2016
Assoc. Prof. Tibor Hlavaty, MD, PhD
IBD Center
Gastroenterology and Hepatology
5th Department of Internal Medicine
Faculty of Medicine
Comenius University and
University Hospital Bratislava
Ruzinovska 6, 826 06 Bratislava
Slovak republic
tibor.hlavaty2@gmail.com
To read this article in full, please register for free on this website.
Benefits for subscribers
Benefits for logged users
Literature
1. Mowat C, Cole A, Windsor A et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011; 60 (5): 571–607. doi: 10.1136/gut.2010.224154.
2. Present DH, Rutgeerts P, Targan S et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Eng J Med 1999; 340 (18): 1398–1405.
3. Hanauer SB, Feagan BG, Lichtenstein GR et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359 (9317): 1541–1549.
4. Sands BE, Anderson FH, Bernstein CN et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Eng J Med 2004; 350 (9): 876–885.
5. Rutgeerts P, Sandborn WJ, Feagan BG et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Eng J Med 2005; 353 (23): 2462–2476.
6. Hyams J, Crandall W, Kugathasan S et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007; 132 (3): 863–873; quiz 1165–1166.
7. Burisch J, Vardi H, Pedersen N et al. Costs and resource utilization for diagnosis and treatment during the initial year in a European inflammatory bowel disease inception cohort: an ECCO-EpiCom Study. Inflamm Bowel Dis 2015; 21 (1): 121–131. doi: 10.1097/MIB.0000000000000250.
8. European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Assessment report: Inflectra (infliximab). [online]. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002778/WC500151490.pdf.
9. Rinaudo-Gaujous M, Paul S, Tedesco ED et al. Review article: biosimilars are the next generation of drugs for liver and gastrointestinal diseases. Aliment Pharmacol Ther 2013; 38 (8): 914–924. doi: 10.1111/apt.12477.
10. Hlavaty T, Letkovsky J. Biosimilars in the therapy of inflammatory bowel diseases. Eur J Gastroenterol Hepatol 2014; 26 (6): 581– –587. doi: 10.1097/MEG.0000000000000 098.
11. Brodszky V, Baji P, Balogh O et al. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ 2014; 15 (Suppl 1): S65–S71. doi: 10.1007/s10198-014-0595-3.
12. Kim J, Hong JA, Kudrin A. 5 year budget impact analysis of CT-P13 (Infliximab) for the treatment of Crohn’s disease in UK, Italy and France. J Crohns Colitis 2015; 9 (Suppl 1): S144–S145.
13. Schwaber J, Cohen EP. Human x mouse somatic cell hybrid clone secreting immunoglobulins of both parental types. Nature 1973; 244 (5416): 444–447.
14. Yoo DH, Hrycaj P, Miranda P et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013; 72 (10): 1613–1620. doi: 10.1136/annrheumdis-2012-203 090.
15. McKoy JM, Stonecash RE, Cournoyer D et al. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion 2008; 48 (8): 1754–1762. doi: 10.1111/j.1537-2995.2008.01749.x.
16. Seidl A, Hainzl O, Richter M et al. Tungsten-induced denaturation and aggregation of epoetin alfa during primary packaging as a cause of immunogenicity. Pharm Res 2012; 29 (6): 1454–1467. doi: 10.1007/s11095-011-0621-4.
17. Park W, Hrycaj P, Jeka S et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 2013; 72 (10): 1605–1612. doi: 10.1136/ annrheumdis-2012-203091.
18. Danese S, Gomollon F. Governing Board and Operational Board of ECCO. ECCO position statement: the use of biosimilar medicines in the treatment of inflammatory bowel disease (IBD). J Crohns Colitis 2013; 7 (7): 586–589. doi: 10.1016/j.crohns.2013.03.011.
19. Gecse KB, Khanna R, van den Brink GR et al. Biosimilars in IBD: hope or expectation? Gut 2013; 62 (6): 803–807. doi: 10.1136/gutjnl-2012-303824.
20. Silverberg MS, Satsangi J, Ahmad T et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19 (Suppl A): 5A–36A.
21. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980; 1 (8167): 514.
22. Vermeire S, Schreiber S, Sandborn WJ et al. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol 2010; 8 (4): 357–363. doi: 10.1016/j.cgh.2010.01.001.
23. Sutherland LR, Martin F, Greer S et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology 1987; 92 (6): 1894–1898.
24. Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 1996; 91 (8): 1571–1578.
25. Lewis JD, Chuai S, Nessel L et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008; 14 (12): 1660–1666. doi: 10.1002/ibd.20520.
26. Targan SR, Hanauer SB, van Deventer SJ et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. N Eng J Med 1997; 337 (15): 1029–1035.
27. Orlando A, Colombo E, Kohn A et al. Infliximab in the treatment of Crohn’s disease: predictors of response in an Italian multicentric open study. Dig Liver Dis 2005; 37 (8): 577–583.
28. Ricart E, Panaccione R, Loftus EV et al. Infliximab for Crohn’s disease in clinical practice at the Mayo Clinic: the first 100 patients. Am J Gastroenterol 2001; 96 (3): 722–729.
29. Farrell RJ, Shah SA, Lodhavia PJ et al. Clinical experience with infliximab therapy in 100 patients with Crohn’s disease. Am J Gastroenterol 2000; 95 (12): 3490–3497.
30. Seminerio JL, Loftus EV Jr, Colombel JF et al. Infliximab for Crohn’s disease: the first 500 patients followed up through 2009. Dig Dis Sci 2013; 58 (3): 797–806. doi: 10.1007/s10620-012-2405-z.
31. Ferrante M, Vermeire S, Katsanos KH et al. Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm Bowel Dis 2007; 13 (2): 123–128.
32. Oussalah A, Evesque L, Laharie D et al. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol 2010; 105 (12): 2617–2625. doi: 10.1038/ajg.2010.345.
33. Gonzalez-Lama Y, Fernandez-Blanco I, Lopez-SanRoman A et al. Open-label infliximab therapy in ulcerative colitis: a multicenter survey of results and predictors of response. Hepatogastroenterology 2008; 55 (86–87): 1609–1614.
34. Rutgeerts P, D’Haens G, Targan S et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 1999; 117 (4): 761–769.
35. Armuzzi A, Pugliese D, Danese S et al. Infliximab in steroid-dependent ulcerative colitis: effectiveness and predictors of clinical and endoscopic remission. Inflamm Bowel Dis 2013; 19 (5): 1065– –1072. doi: 10.1097/MIB.0b013e3182802 909.
36. Schnitzler F, Fidder H, Ferrante M et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut 2009; 58 (4): 492–500. doi: 10.1136/gut. 2008.155812
37. Teshima CW, Thompson A, Dhanoa L et al. Long-term response rates to infliximab therapy for Crohn’s disease in an outpatient cohort. Can J Gastroenterol 2009; 23 (5): 348–352.
38. Kang Y-S, Moon HH, Lee SE et al. Clinical experience of the use of CT-P13, a biosimilar to infliximab in patients with inflammatory bowel disease: a case series. Dig Dis Sci 2015; 60 (4): 951–956. doi: 10.1007/s10620-014-3392-z.
39. Jung YS, Park DI, Kim YH et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol 2015. doi: 10.1111/jgh.12997.
40. Jarzębicka D, Płocek A, Sieczkowska J et al. First observations of the use of biosimilar infliximab for treatment of ulcerative colitis in paediatric population. J Crohns Colitis 2015; 9 (Suppl 1): S307–S308.
41. Sieczkowska J, Banaszkiewicz A, Płocek A et al. Assessment of safety and efficacy of biosimilar infliximab in children with Crohn disease: a preliminary report. J Crohns Colitis 2015; 9 (Suppl 1): S295.
42. Jarzębicka D, Banaszkiewicz A, Płocek A et al. Preliminary assessment of efficacy and safety of switching between originator and biosimilar infliximab in paediatric Crohn disease patients. J Crohns Colitis 2015; 9 (Suppl 1): S224–S225.
43. Gecse K, Farkas K,Lovasz B et al. Biosimilar infliximab in inflammatory bowel diseases: first interim Results from a prospective nationwide observational cohort. J Crohns Colitis 2015; 9 (Suppl 1): S234–S235.